Abdominal Aortic Aneurysms: Diagnosis and Management
Sean J. English
Jonathan L. Eliason
Gilbert R. Upchurch Jr
INTRODUCTION
Abdominal aortic aneurysms (AAAs) primarily affect the elderly and have a high mortality rate if left untreated. Encounters with this disease will become more frequent as society ages during the 21st century, as evidenced by the observation that AAA accounts for approximately 150,000 inpatient hospital admissions per year in the United States. Data from the CDC National Vital Statistics on deaths from the year 2006 demonstrated that aortic aneurysm constituted the 16th leading cause of death in all patients aged 65 to 85 years. Aortic disease represented the 15th leading cause of death for Caucasian males of the same age group. The year 2000 National Hospital Discharge Summary reports more than 30,000 open operations for the repair of AAA were performed in the United States. Dimick et al. using Medicare data from all hospitals in the United States from 2001 to 2003 that performed AAA surgery documented 54,302 open repairs that were performed during that time period, while 26,750 endovascular repairs were performed during that same time period. Better defining those patients who are most at risk for AAA development is therefore an important undertaking.
Risk Factors
Atherosclerosis, a common finding in patients with AAAs, is believed to be a secondary rather than a primary etiologic factor in AAA development and may potentiate its development by further compromising the structure of the aortic wall. Age, male gender, smoking, hypertension, and the presence of chronic obstructive pulmonary disease (COPD) place patients at increased risk for the development of AAAs. Genetic factors also appear important in AAA development, with 15% of patients having a first-degree relative with an AAA (Table 12.1). For example, a decrease in aortic wall type III collagen has been noted in individuals with a firstdegree relative with an AAA, compared to those without a family history of AAAs. Increases in the frequency of the Hp-2-1 haptoglobin phenotype, as well as the Kell positive and MN blood groups, have been noted in patients with AAAs. By contrast, there is a decrease in the incidence of AAAs in patients with type A Rh-negative blood group. A number of genetic polymorphisms have been associated with AAAs. However, since most AAAs are degenerative in nature, a polygenic inheritance pattern is likely. Rarer causes of AAAs include trauma, dissections, vasculitis, and infection (Table 12.1).
TABLE 12.1 VARIOUS ETIOLOGIES OF AAAs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pathogenesis
AAAs are characterized by marked inflammation and an imbalance between the production and the degradation of structural extracellular matrix proteins. Disruption and degradation of medial elastin and collagen is a particularly prominent feature of AAA formation. In this regard, increased local production of enzymes that degrade elastin and collagen, namely the matrix metalloproteinases, has been proposed as being pivotal in vessel wall initiation and clinical progression of aneurysmal disease.
Clinical Features
Most AAAs are asymptomatic and over 80% are located in the infrarenal aorta. Normal aortic pulsation is roughly the width of the patient’s thumb. An aortic aneurysm may be suspected when the aortic pulse is expansile and larger than two centimeters. Extension of the pulsations to the xiphoid or costal margins should make one suspect a thoracoabdominal or suprarenal aortic aneurysm. Presence of tenderness over an aneurysm is an ominous sign and may indicate impending rupture. Palpation of the lateral borders of the aorta between one’s fingertips on abdominal examination may suggest the presence of a large pulsatile mass. However, prominent anterior pulsations alone are more likely to be due to an ectatic, nonaneurysmal aorta than an AAA. It is often difficult to palpate the infrarenal aorta in the epigastrium because the aortic bifurcation is at the level of the umbilicus. Thus, physical examination alone is unreliable in most patients, especially if obese. In one series of patients with known AAAs, experienced clinicians were unable to make the diagnosis in close to half the patients with the disease.
Concomitant Aneurysmal Disease
Patients with AAAs may also have aneurysms of the femoral or popliteal arteries. A study in patients with AAAs from the University of Michigan
documented a 14% incidence of aneurysms of the femoral and popliteal arteries, all occurring in hypertensive male patients. The presence of these extremity aneurysms may serve as a clue to the existence of an AAA. In addition, it is important to recognize that patients with femoral and popliteal artery aneurysms have approximately an 80 and 60% chance of having an AAA, respectively.
documented a 14% incidence of aneurysms of the femoral and popliteal arteries, all occurring in hypertensive male patients. The presence of these extremity aneurysms may serve as a clue to the existence of an AAA. In addition, it is important to recognize that patients with femoral and popliteal artery aneurysms have approximately an 80 and 60% chance of having an AAA, respectively.
Clinical Features of a Ruptured Abdominal Aortic Aneurysm
The classic triad of acute hypotension, back or abdominal pain, and a palpable abdominal mass is rarely seen. The patient with an unsuspected AAA may present with a clinical picture that suggests exacerbation of chronic back pain. A more acute presentation with the acute onset of abdominal, flank, or back pain radiating to the groin may be secondary to AAA expansion or rupture. Without imaging studies or a high index of suspicion, this pain may be confused with diverticulitis, renal colic, irritable bowel syndrome, inflammatory bowel disease, ovarian torsion, or appendicitis. In the hemodynamically stable patient with symptoms consistent with the diagnosis of an AAA, the use of abdominal ultrasonography (US) may be performed expeditiously as it confirms the suspected diagnosis of a ruptured AAA (rAAA), requires no transport time, and is quite sensitive. CT angiography in a stable patient when the diagnosis is uncertain is also now routinely performed, with the advent of endovascular aortic repair (EVAR). Patients with severe hypotension, unrelenting abdominal or back pain, and a suspected rAAA should be transported urgently to the endovascular suite where an open or an endovascular approach may be used.
DIAGNOSIS
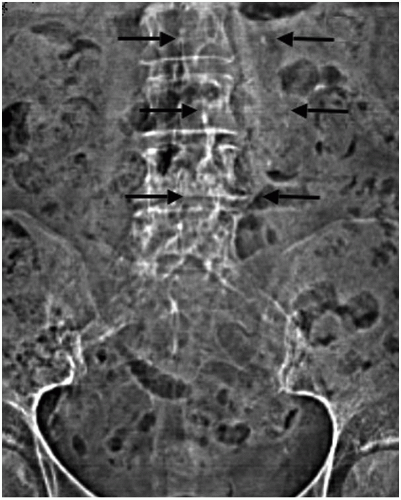
Once an AAA is suspected, a logical diagnostic algorithm should be followed (Fig. 12.1). Plain abdominal or lumbosacral radiographs performed in either the anterior-posterior (AP) or the lateral projections done for other reasons, such as to examine the lumbar spine, may suggest the presence of an AAA by demonstrating a rim of calcification in the outer aortic wall (Fig. 12.2).
Ultrasound
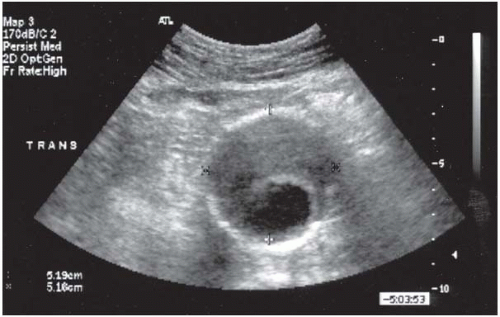
The most useful means to establish the diagnosis of an AAA is duplex US. US is a noninvasive, inexpensive test that provides reliable and reproducible measurements of the aortic diameter (Fig. 12.3). US correlates closely with operative measurements of AAA, and interobserver variability of less than 5 mm has been demonstrated in 80% of AP measurements. Errors and limitations with US are most often attributed to inexperienced technicians, lack of interpretive skills, or excessive bowel gas.
Computed Tomography Angiography
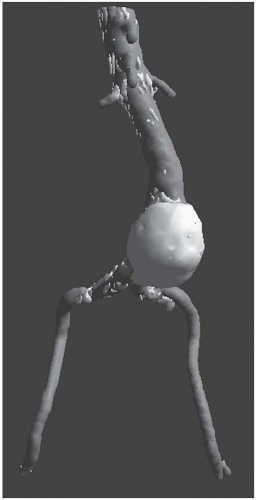
Computed tomography angiography (CTA) should be ordered if intervention is planned, as CT is highly predictive of AAA size, with interobserver variability of less than 5 mm existing in 91% of AP measurements. Importantly, CT is superior to US in assessing AAA wall integrity, the
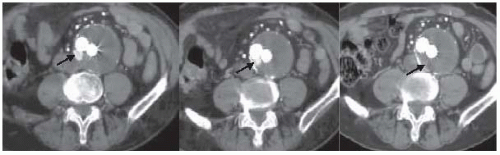
location and amount of calcification within vessel walls, venous anomalies, retroperitoneal blood, aortic dissection, and infection or inflammation, as well as the proximal and distal extent of the aneurysm. CT may also demonstrate other intra-abdominal pathology (presence of various renal, adrenal, and gastrointestinal abnormalities) that may be important during planning for either an open or an endovascular approach. Limitations of CT include (a) need for nephrotoxic iodinated contrast administration, (b) increased radiation exposure, and (c) increased cost. Spiral or helical CTA provides excellent resolution and coronal reconstructions (Fig. 12.4). In addition to providing an anatomical roadmap prior to repair of AAAs, CT is the study of choice for assessing for postoperative endoleak following endovascular AAA repair (Fig. 12.5). Several recent studies have also suggested that US with power Doppler or various nonionic contrast agents (i.e., microbubbles) may also be used to follow AAA size and the presence of an endoleak (Fig. 12.6). This approach appears to be most useful in the setting of decreasing AAA size with prior CT evidence of no endoleak.
location and amount of calcification within vessel walls, venous anomalies, retroperitoneal blood, aortic dissection, and infection or inflammation, as well as the proximal and distal extent of the aneurysm. CT may also demonstrate other intra-abdominal pathology (presence of various renal, adrenal, and gastrointestinal abnormalities) that may be important during planning for either an open or an endovascular approach. Limitations of CT include (a) need for nephrotoxic iodinated contrast administration, (b) increased radiation exposure, and (c) increased cost. Spiral or helical CTA provides excellent resolution and coronal reconstructions (Fig. 12.4). In addition to providing an anatomical roadmap prior to repair of AAAs, CT is the study of choice for assessing for postoperative endoleak following endovascular AAA repair (Fig. 12.5). Several recent studies have also suggested that US with power Doppler or various nonionic contrast agents (i.e., microbubbles) may also be used to follow AAA size and the presence of an endoleak (Fig. 12.6). This approach appears to be most useful in the setting of decreasing AAA size with prior CT evidence of no endoleak.
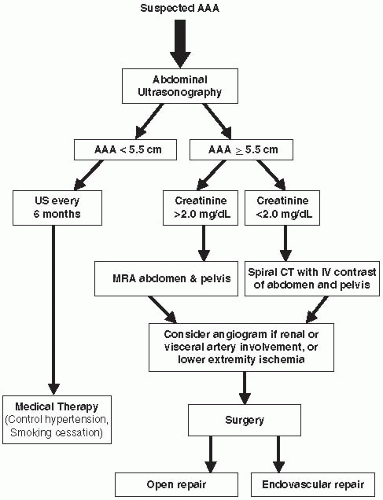 FIGURE 12-1. Algorithm for patient suspected of having an AAA. (US, ultrasound; MRA, magnetic resonance angiography; CTA, computed tomography angiography.) |
Magnetic Resonance Angiography
Magnetic resonance angiography (MRA) using nonnephrotoxic gadolinium with a breath-hold technique is comparable to CT scanning for AAA measurements. Images are based on T1 relaxation rather than blood flow, which means that slow flow in AAAs does not adversely affect the image. An earlier reported experience with 43 AAAs revealed that MRA correctly identified maximum AAA diameter and carried 94 and 98% sensitivity and specificity, respectively, for identifying significant stenoses of the splanchnic, renal, or iliac arteries. MRA limitations include the inability to scan
patients who have pacemakers, defibrillators, or claustrophobia, and the fact that images may be obscured by artifacts caused by metallic objects including certain vascular stents. Disadvantages of MRA also include its inability to image calcified plaque, a finding important in endovascular interventions. However, MRA may be more sensitive than CTA for the detection of endoleaks after endovascular aortic aneurysm repair (EVAR).
patients who have pacemakers, defibrillators, or claustrophobia, and the fact that images may be obscured by artifacts caused by metallic objects including certain vascular stents. Disadvantages of MRA also include its inability to image calcified plaque, a finding important in endovascular interventions. However, MRA may be more sensitive than CTA for the detection of endoleaks after endovascular aortic aneurysm repair (EVAR).
Catheter-based Angiography
Catheter-based angiography with digital subtraction techniques is usually obtained when concomitant atherosclerotic vascular involvement is suspected, and treatment is planned prior to operative repair. Intravascular US (IVUS) may also be used concurrently when performing angiography. IVUS may be especially useful in assessing the size of the aortic neck, as most believe that CT tends to oversize aortic neck diameter. Angiography and IVUS may be useful in identifying the cephalad extent of the AAA, the number and location of renal arteries, the state of the splanchnic arteries, and the status of the iliac arteries, as well as the presence of occlusive disease in the lower-extremity arteries. Complications of angiography include bleeding or arterial occlusion at the catheterization site, atheroembolism, and impairment of renal function due to iodine-induced contrast nephrotoxicity.
Management of the Patient with Abdominal Aortic Aneurysm
The most serious complication of an AAA is continued expansion until aneurysm rupture occurs. Therefore, it is important to recognize factors contributing to AAA rupture. In accordance with the law of Laplace, a
geometric increase in aortic wall pressure occurs with linear increases in AAA size. Thus, an increase in aortic diameter from 2 to 4 cm induces, not a twofold, but a fourfold increase in the pressure/cm2 on the aortic wall. Rupture is directly proportional to aortic wall pressure. It is also known
that aortic elastic tissue loses its integrity with age. Acquired (smoking, hypertension), genetic factors, and patient characteristics (increasing age, male gender) hasten this process and add to the risk of accelerated AAA expansion. Aneurysm expansion greater than 6 mm over a 12-month time period suggests an unstable AAA and is a soft indication for early intervention in AAAs approaching 5 cm in diameter. An intact asymptomatic AAA with a diameter of 5 cm is generally recognized to carry a risk or rupture of 10% or less over 2 to 3 years. The risk of rupture for smaller aneurysms, 3 to 5 cm in size, is less well defined. However, greater anterior to posterior diameter, COPD, and diastolic hypertension all independently increase the
chance of AAA rupture. Thus, the presence of a 5-cm AAA in a patient with diastolic hypertension and COPD is a cause for concern, with a predicted rupture rate of more than 30% in a year, compared to a 3-cm AAA in a normotensive patient without COPD, which has a rupture rate of only a few percentages over 5 years.
geometric increase in aortic wall pressure occurs with linear increases in AAA size. Thus, an increase in aortic diameter from 2 to 4 cm induces, not a twofold, but a fourfold increase in the pressure/cm2 on the aortic wall. Rupture is directly proportional to aortic wall pressure. It is also known
that aortic elastic tissue loses its integrity with age. Acquired (smoking, hypertension), genetic factors, and patient characteristics (increasing age, male gender) hasten this process and add to the risk of accelerated AAA expansion. Aneurysm expansion greater than 6 mm over a 12-month time period suggests an unstable AAA and is a soft indication for early intervention in AAAs approaching 5 cm in diameter. An intact asymptomatic AAA with a diameter of 5 cm is generally recognized to carry a risk or rupture of 10% or less over 2 to 3 years. The risk of rupture for smaller aneurysms, 3 to 5 cm in size, is less well defined. However, greater anterior to posterior diameter, COPD, and diastolic hypertension all independently increase the
chance of AAA rupture. Thus, the presence of a 5-cm AAA in a patient with diastolic hypertension and COPD is a cause for concern, with a predicted rupture rate of more than 30% in a year, compared to a 3-cm AAA in a normotensive patient without COPD, which has a rupture rate of only a few percentages over 5 years.
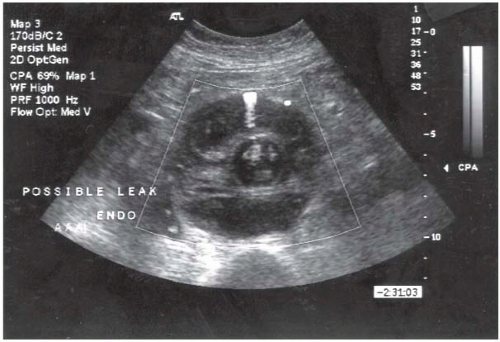 FIGURE 12-6. Duplex ultrasound performed postoperatively following endovascular AAA repair showing a type 2 (branch vessel) endoleak. |
The risk of death following AAA rupture depends on performing an emergent and expeditious intervention. Attention to repairing the rAAA is paramount, and wasted time repairing small iliac artery aneurysms should be discouraged. Unfortunately, nearly 60% of patients with rAAAs die before reaching a hospital, and only 50% of the remainder survive an emergent operation. Thus, AAA rupture carries an 80% mortality rate.
Surgical Therapy
Elective operative intervention by open aortic repair (OAR) or an EVAR lessens the likelihood of death due to a rAAA. Conventional open repair in elective circumstances in a large population-based study from the state of Michigan carried a 5.6% mortality rate. Surprisingly, women fared worse than men following aneurysmectomy with operations for both intact and ruptured aneurysms. The elective mortality rate over an 11-year time period in women was 10.7% compared to 6.8% in men in this experience. Risk factors found to be predictive of a poor outcome and an increased inhospital mortality following elective OAR include female gender, increased age (over 65 years), aneurysm rupture, and low volumes of aortic surgery. In fact, based only on age, gender, and hospital volume, up to an eightfold increase in mortality has been predicted.
Conventional Surgical Treatment of Intact Abdominal Aortic Aneurysms
A rapid operation is important for successful OAR, as an operation lasting longer than 5 hours is independently associated with an increase in mortality and significant cardiopulmonary complications. Other factors associated with poor surgical outcome include operative hypothermia, excessive blood loss, coagulopathy, and the need for supraceliac aortic cross clamping. Surgical approaches are individualized for each patient, with transperitoneal or retroperitoneal aortic exposure based on both the surgeon’s preference and the aortic pathology. A transperitoneal approach is preferred when there is a need to revascularize the right kidney or if aneurysmal disease extends into the right iliac artery. Certain anatomic and clinical circumstances where a retroperitoneal approach is preferable include obese patients and those with multiple prior laparotomies and a “hostile” abdomen. Although the retroperitoneal approach does not significantly decrease mortality or major cardiopulmonary morbidity, multiple randomized trials suggest it expedites return of postoperative bowel function.
In the past, thrombotic and bleeding complications due to clamping and unclamping the aorta were the major complications following OAR. To address these issues, prior to occluding, the aorta patients are
systemically anticoagulated with intravenous heparin, and the aorta is clamped distally prior to clamping proximally. The aneurysm is then incised, intraluminal clot is removed, and back-bleeding lumbar arteries are oversewn, before the aortic graft is sewn in place. The prosthetic grafts currently used are either woven or knitted Dacron or extruded polytetrafluoroethylene. After the graft is in place and blood flow is restored to the pelvis and the lower extremities, it is important that the graft be covered with the aneurysm sac and the retroperitoneum, so as to prevent contact with the intestines. Such contact may lead to later graft-enteric erosion, which is considered a life-threatening complication requiring graft removal.
systemically anticoagulated with intravenous heparin, and the aorta is clamped distally prior to clamping proximally. The aneurysm is then incised, intraluminal clot is removed, and back-bleeding lumbar arteries are oversewn, before the aortic graft is sewn in place. The prosthetic grafts currently used are either woven or knitted Dacron or extruded polytetrafluoroethylene. After the graft is in place and blood flow is restored to the pelvis and the lower extremities, it is important that the graft be covered with the aneurysm sac and the retroperitoneum, so as to prevent contact with the intestines. Such contact may lead to later graft-enteric erosion, which is considered a life-threatening complication requiring graft removal.
Endovascular Surgical Treatment of Intact Abdominal Aortic Aneurysms
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree