Chapter 4
Abdominal Aortic Aneurysms
David A. Sidloff, Nikesh Dattani and Matthew J. Bown
Department of Cardiovascular Sciences, University of Leicester, UK
Introduction
The infra-renal abdominal aorta is the commonest site for arterial aneurysms, the vast majority of which are fusiform in shape (Figure 4.1). Abdominal aortic aneurysms (AAAs) cause approximately 4000 deaths per annum in England and Wales, largely due to rupture. The number of deaths due to AAAs rose throughout the 20th century; however, over the last decade, an unexplained, steady decline in AAA mortality has been noted in most developed countries (Figure 4.2). Despite this, the mortality rate following the rupture of AAAs in the community approaches 90%, making it an important cause of mortality. The true mortality of AAAs may even be higher as many patients dying from AAA rupture do not undergo formal post-mortem examination.
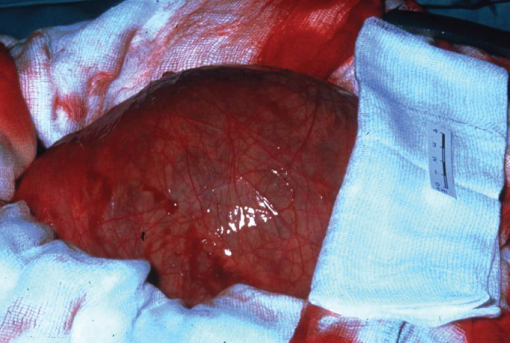
Figure 4.1 Operative photograph of an intact AAA.
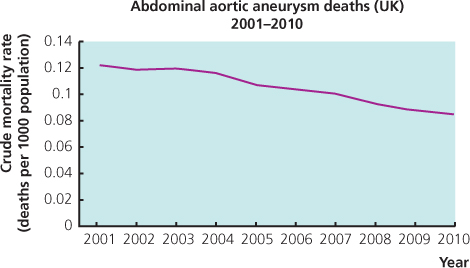
Figure 4.2 Death rates due to abdominal aortic aneurysm 2001–2010 in the United Kingdom. Data from the World Health Organisation mortality database (http://apps.who.int/healthinfo/statistics/mortality/whodpms/).
Definition
The true definition of aneurysmal arterial dilatation is an increase in vessel diameter of 50% or more in relation to an adjacent normal arterial segment. For practical purposes (in the case of the abdominal aorta), a measurement of greater than 3 cm in any axial diameter is taken to be diagnostic for AAAs. Normal infra-renal diameter is 2.0–2.4 cm in males and 1.6–2.2 cm in females.
Epidemiology
In population screening studies of men over the age of 65, estimates for the prevalence of AAAs greater than 3 cm in size vary from 1.7% in the United Kingdom to 8.7% in New Zealand. The prevalence of AAAs is lower in women at approximately 1.5%. AAA is primarily a disease of white Europeans. They are extremely rare in Asian populations. This appears to be a genetic effect as Asian populations in the United Kingdom have a much lower incidence of AAAs than UK Caucasians. The incidence of AAAs also seems to be shifting towards an older population with males >75 years old now being the group most at risk.
Risk factors for abdominal aortic aneurysms (Box 4.1)
Smoking is the most significant risk factor for AAAs and is the only modifiable risk factor that has been associated with the development, expansion and rupture of AAAs. This has been confirmed by several prospective studies that have demonstrated a dose-dependent relationship and a 2.6 to 9.0 fold increase in risk of AAAs in smokers compared to non-smokers. The duration of smoking rather than amount smoked has a more significant effect on the risk of AAA formation and the risk of AAA only gradually reduces over time after smoking cessation.
Other risk factors have been identified for the formation of AAAs, although the strength of these associations is not as well defined as for smoking. Hypertension, hyperlipidaemia and an increased body mass index have all been associated with AAA development in some studies but other studies have failed to demonstrate this association. Interestingly, diabetes mellitus, which is traditionally a strong cardiovascular risk factor, seems to protect against both the development and the growth of AAAs.
Non-environmental risk factors for AAAs are age, race and gender. AAA rarely affects patients below the age of 40, and with increasing age, the prevalence of AAA increases. Above the age of 65, each 7-year age increment confers a 1.5-fold increase in the risk of AAAs. AAA is extremely rare in Asian populations and is predominately a disease of males, the prevalence being nearly 6-fold greater in males than females. Although this sexual dimorphism exists among a number of cardiovascular diseases, it is not fully understood. There is a strong genetic component in the aetiology of AAAs, which appears to be due to multiple small-effect genetic loci rather than a single gene defect and studies to define the genetic risk factors for AAAs are ongoing.

Figure 4.3 Photograph of a very thin patient with a large AAA that could be both seen and felt by the patient.
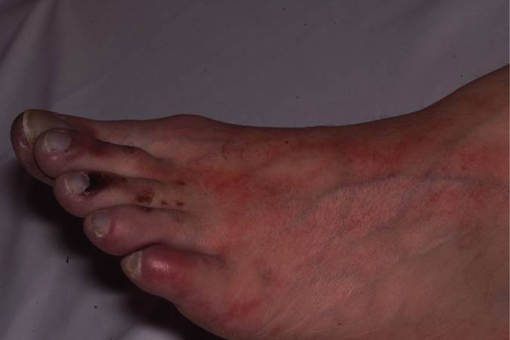
Figure 4.4 Focal infarction of areas of the first, second and third toes of the left foot in a patient with an AAA due to micro-embolisation of thrombus from within the aneurysm sac (trash-foot).
Symptoms, signs and complications
The majority of AAAs (90%) are asymptomatic until the onset of complications, the most serious of which is rupture. Thin patients may notice a pulsatile swelling in their abdomen (Figure 4.3). The complications of any aneurysm are due to rupture, thrombosis or embolism. Acute thrombosis can occur in abdominal aneurysms but is more common in aneurysms at other sites such as the popliteal artery. Embolisation of thrombus from AAAs causing acute distal ischaemia (trash-foot) is more common than acute thrombosis (Figure 4.4). Together acute thrombosis or embolism only occur in 3–5% of patients with AAAs.
The classical presenting triad of ruptured abdominal aortic aneurysms (RAAAs) is pain, either abdominal or back, hypotension and a pulsatile abdominal mass. As only approximately 25% of patients with RAAAs present with all the three signs, a high index of suspicion for RAAAs is essential in any patient who presents with any one of these symptoms and signs. Some patients may develop signs related to retroperitoneal haemorrhage such as flank or peri-umbilical bruising (Figure 4.5).
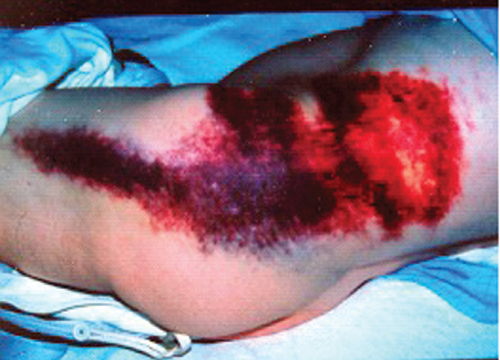
Figure 4.5 Flank bruising due to retroperitoneal blood from a ruptured AAA tracking laterally into the subcutaneous tissues. A rare sign of ruptured AAAs.
Diagnosis
Clinical examination is not a reliable tool for the diagnosis of AAAs. The simplest diagnostic test for AAAs is ultrasound that has a sensitivity and specificity approaching 100%. This also has the advantage of not requiring ionising radiation and because ultrasound technology is portable, it lends itself to being used as a community screening tool. Computerised tomography (CT) scanning has a similar sensitivity and specificity for the diagnosis of AAAs as ultrasound; however, CT also provides additional information regarding the morphology and anatomical relationships of the AAA (Figure 4.6). This information is essential in planning surgery and determining suitability for endovascular repair. Magnetic resonance imaging (MRI) provides similar information as CT but with the disadvantage of additional cost and limited patient acceptability. The main advantage of MRI is in the avoidance of ionising radiation.
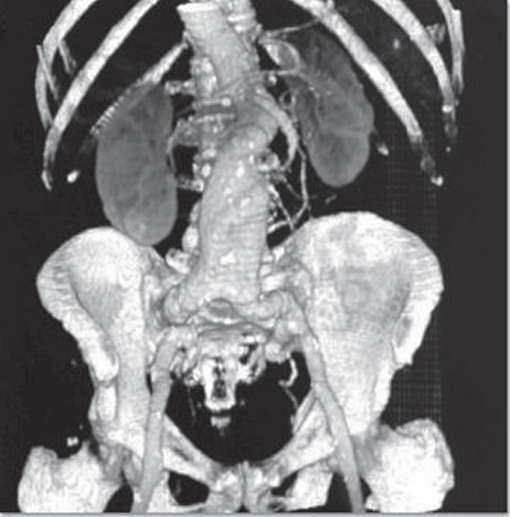
Figure 4.6 Three-dimensional computed tomography (CT) reconstruction of an infra-renal AAA. The kidneys and bony skeleton have been included in the reconstruction for reference.
In emergency situations, ultrasound scanning is useful to confirm the presence or absence of an AAA but cannot provide any information on the likelihood of AAA rupture. It is of most use in haemodynamically stable patients in whom the diagnosis of AAA is not suspected but needs to be excluded. CT scanning is more than 90% sensitive and specific for the detection of ruptured AAAs. Modern CT scanners can acquire images in as little as 30 s and thus cause little delay in transferring patients to theatre for surgery. The extra information provided and the possibility of assessment for endovascular repair are of significant benefit.
Risk of rupture
Before surgery for AAAs was developed in the second half of the 20th century, studies of patients with AAA who were left untreated demonstrated that 81.1% will die within 5 years compared to only 20.9% of the normal age-matched population. After 8 years, only 10% of those with AAA are alive compared to 65% of the normal population. In patients with AAA unfit for surgery, a quarter will die due to AAA rupture and the majority of these ruptures (80%) occur within 3 years of diagnosis.
AAA size is the single most important factor in determining the risk of rupture; however, rupture rates of small AAAs have been shown to be 2-fold higher in current smokers and 4-fold higher in women. Studies of ruptured AAAs have demonstrated that the median size is usually above 8 cm in diameter, whereas only a very small proportion of screening detected asymptomatic cases (0.16%) with greater than 6 cm in diameter. Autopsy studies have found that RAAAs are significantly larger than incidentally found intact AAAs (8 cm vs 4 cm). This implies that aneurysms are at higher risk of rupture as they increase in size.
Patients with large aneurysms (more than 5.5 cm) are usually offered surgery, provided they are fit enough. Patients with smaller aneurysms are a more difficult group to manage. Two large randomised controlled trials, one in the United Kingdom and one in the United States, have compared the strategy of early elective open surgical repair compared with surveillance in patients with small AAAs (4.0–5.5 cm). These studies demonstrated no survival advantage in patients undergoing early elective open surgery with similar findings after 12 years of follow-up in the UK Small Aneurysm Trial. Furthermore, the Comparison of Surveillance Versus Aortic Endografting for Small Aneurysm Repair (CAESAR) trial demonstrated similar findings with no advantage to early endovascular aneurysm repair (EVAR). Patients with asymptomatic AAAs that are less than 5.5 cm are, therefore, usually monitored ultrasonographically rather than offered surgery. Because of this relationship between size and risk of rupture, AAA size becomes the critical factor dictating clinical management.
Screening for AAAs
Screening programmes to detect AAAs have now been set up in many developed countries. The rationale behind AAA screening is that AAAs are often asymptomatic before rupture, have a long latent period and can be detected during an early phase in the disease using a test that is sufficiently sensitive, acceptable and cost effective. Furthermore, the mortality associated with AAA repair is significantly lower in the elective rather than emergency setting. The usefulness of AAA screening was first addressed in the Multicentre Aneurysm Screening Study (MASS) in 2002 that demonstrated a 42% risk of death reduction in a group of 65- to 74-year-old men screened for AAA. More recently, long-term data from this study suggests that the benefit of screening men aged 65–74 for AAA is maintained up to 13 years post screening and is highly cost effective in terms of cost per quality-adjusted life-year gained.
Surveillance
Following detection of a small AAA (<5.5 cm), patients are usually not offered surgery as the risks of surgery outweigh the current risk of rupture. However, because it is known that small AAAs grow with time, the risk of rupture also increases and it is important to provide surveillance for these patients. A recent meta-analysis of individual patient data by the RESCAN collaborators suggested that for each 0.5 cm increase in AAA diameter, growth rates increased on average by 0.59 mm per year and rupture rates increased by a factor of 1.91. A 3.5-cm AAA will on average take 6.2 years to reach 5.5 cm, whereas a 4.5 cm AAA will only take 2.3 years; therefore, the growth rate of AAAs is not linear. Currently, in the United Kingdom, those with 30–44 mm AAAs are offered yearly surveillance, whereas those with 45–54 mm AAAs are followed up every 3 months. In the future, patient-specific surveillance may become the standard of care.
Treatment
Currently, the only curative treatment for AAAs is surgery (Box 4.2). Traditionally, this consisted of open abdominal repair; however, since the early 1990s, endovascular techniques have become widely available and are rapidly advancing. While AAA is the main focus of this chapter, it should not be forgotten that each patient is likely to have significant cardiovascular risk factors and there is the opportunity to address these. Best medical therapy including antiplatelet agents, statins and antihypertensive medications in addition to smoking cessation advice should be considered in all patients. Improvements in the control of these common cardiovascular risk factors have likely contributed to the current decline in AAA-related mortality being observed in many developed economies.

Full access? Get Clinical Tree


