Abdominal Aortic Aneurysm
Martin Köcher
Petr Utíkal
Infrarenal aneurysm of the abdominal aorta (AAA) afflicts 1% to 6% of the population >60 years of age, and its incidence is constantly rising.1,2 When left untreated, AAA may be fatal. Within 1 year of establishment of the diagnosis, 50% of AAAs rupture, and within 5 years this number rises to 90%.3
Whereas the mortality of patients undergoing acute operations for the rupture of an aneurysm approaches 70%, that of elective operative treatment ranges up to 5% in most studies.4,5,6,7,8,9 Efforts are thus concentrated on the elective treatment of all identified AAAs. The aim of AAA treatment is to prevent its rupture by shutting off the aneurysmal sack from the circulation.
The standard treatment of AAA is open surgical repair. The technique and strategy of resecting the AAA, replacing the aorta with an artificial vascular prosthesis, was developed and has been employed in clinical practice since the 1950s.10,11
However, open surgery is not a good option for patients with high surgical risk, since their mortality is 19% and morbidity 40%.12,13 In fact, elective surgical procedure may even be contraindicated in some patients because of coexisting morbidities.
In 1991, Parodi et al.14 introduced endovascular aneurysm repair (EVAR), which now represents a viable alternative to open surgery.
Clinical Description
Etiology and Epidemiology of Abdominal Aortic Aneurysm
Dimensions of the normal supraceliac, suprarenal, and infrarenal aorta have been recently reviewed.15 AAA is defined as a distension of the infrarenal aorta by >50% (or 1.5 times) com-pared with a corresponding healthy, age- and gender-matched population.16
AAA affects 1% to 6% of the population aged >60 years,1,2 and the incidence rises by approximately 0.15% annually. The incidence may be also increasing because of aging populations and the greater availability of ultrasound screening.17 The incidence ratio of AAA in men and women is 4-5:1.
According to its pathological morphology, AAA is considered a true aneurysm, because it comprises all layers of the arterial wall, most commonly forming into a fusiform shape. Aneurysms with a diameter <5 cm are considered small, and those >6.5 cm large.15
The most common cause of AAA is a dilation type of atherosclerosis (95%). Less common are AAAs of infectious or inflammatory origin, or those associated with connective tissue diseases.18 The process of AAA formation is multifactorial. Apart from the general risk factors of atherosclerosis, genetic disposition, autoimmunity, and hemodynamic factors all play roles in its formation.18 AAA is 1.5 times more frequent in hypertensive patients and in those with manifested atherosclerotic peripheral arterial disease.19,20 A significantly higher incidence of AAA has been observed in smokers (eight times).21 A common histopathologic element is the inflammatory reaction within the aortic wall that leads to the destruction of the intercellular matrix (particularly elastin) and the remodeling of collagen, which consequently leads to the loss of aortic wall elasticity and rigidity.15
Clinical Signs of Abdominal Aortic Aneurysm
In up to 75% of patients, AAA remains asymptomatic and presents acutely with ruptures.22 In patients with clinical symptoms, the manifestation may be nonspecific; abdominal or back pain may be clinical signs of an already unstable or penetrating aneurysm. At present, the majority of aneurysms are discovered during examinations (e.g., ultrasonography, USG; computed tomography, CT; or magnetic resonance, MR, imaging) that were indicated for other reasons. According to their presentation, the
aneurysms are classified as asymptomatic and symptomatic with or without a rupture.15
aneurysms are classified as asymptomatic and symptomatic with or without a rupture.15
The prognosis of a patient with AAA is unfavorable. In men older than 55 years, AAA is the 10th most frequent cause of death. The rupture of an aneurysm is the most severe complication and, when untreated, it is usually lethal. Overall, of all sudden deaths in the age group 18-70 years, 4.2% in men and 1.2% in women are related to a ruptured AAA.23 It is generally accepted that within 1 year of the established diagnosis, approximately half of the cases of AAA rupture and within 5 years this number rises to 90%. The average interval between an established diagnosis and the rupture of an untreated aneurysm has been reported to be 16 months.3 The risk of rupture increases with the size of the AAA, with the transverse diameter of the AAA being the most powerful predictor of rupture. In small aneurysms (<5 cm) the yearly risk of rupture is 6%, in aneurysms with a diameter of 7 cm it is 23%, and in those >10 cm it is 60%.24 According to other authors, the annual risk of rupture approaches 0% for aneurysms <4 cm in diameter, whereas for aneurysms with a diameter of 4 to 4.9 cm, 5 to 5.9 cm, 6 to 6.9 cm, 7 to 7.9 cm, and 8 cm and larger, the annual risk of rupture ranges from 0.5% to 5%, 3% to 15%, 10% to 20%, 20% to 40%, and 30% to 50%, respectively.25,26,27
However, there is not always a linear correlation between the size of an AAA and the risk of aneurysmal rupture. Even a small aneurysm can rupture.28,29 Furthermore, 80% of aneurysms expand. Among these, 20% expand >0.5 cm per year, and this rapid growth also increases the risk of rupture.30 Other risks of rupture include cigarette smoking,31,32 hypertension,32 positive family history,33,34 chronic obstructive pulmonary disease,32,35 female gender (the risk of rupture is three times higher in women than it is in men),32 and a saccular shape of aneurysm.36,37
Patients with AAA are included in the group of so-called “vascular surgically ill” patients and the majority of these patients have a high operative risk. Operative risk factors include creatinine >1.8 mg/dL, congestive heart failure, evidence of myocardial ischemia on ECG, pulmonary dysfunction, age, and female gender.38 In addition, 75% of AAA patients have at least two severe comorbidities, including peripheral arterial disease frequently associated with a generalized vascular disease involving the coronary, renal, and cerebral circulation. Coronary artery disease may be present in as many as 80% of patients. Most patients are smokers with respiratory disorders, hypertension, and diabetes mellitus. The presence of severe coexistent diseases significantly restricts the surgical options for AAA treatment and plays a significant role in the perioperative morbidity and mortality.3,38
Treatment Strategies for Abdominal Aortic Aneurysm
Surgical Treatment
The standard surgical treatment technique is resection of the aneurysmal sack and its replacement with an artificial vascular prosthesis that is sutured onto the aorta with vascular stitches.10,11 This invasive procedure is associated with hemodynamic compromise due to opening the abdominal wall (laparotomy) and temporary clamping of the infrarenal aorta.40
The outcome of surgical treatment of AAA depends primarily on the emergency versus elective status of the procedure and on the morbidity of the patient. The reported mortality of emergency surgical treatment for ruptured AAA ranges between 23% and 70%. In contrast, the reported mortality of elective surgical treatment of AAA is approximately 2% to 8% at present.4,5,6,7,8,9,16,41 Surgical AAA repair is associated with considerable morbidity, including cardiac morbidity ranging between 10% and 12%, pulmonary morbidity ranging between 5% and 10%, and renal morbidity ranging between 5% and 7%; these figures pertain to elective surgical AAA treatment in patients with a low operative risk. In patients with high operative risk, the mortality and cardiopulmonary morbidity is considerably higher, namely, 19% and 40%, respectively.12,13 To improve the early outcome of elective AAA surgery, stricter selection criteria would be required. Prior to EVAR, this meant exclusion of a number of patients eligible for elective treatment.42
Endovascular Treatment
EVAR is today considered an important alternative to open surgery for AAA.43 The principle of EVAR is to shut off the aneurysm from the circulation by bridging it with a stent-graft prosthesis introduced and placed endoluminally. The stent-graft prosthesis is introduced in a folded state from a common femoral artery via the pelvic vessels into the aorta using a guidewire-based delivery system. In the aorta, the stent graft is released and anchored at the sites above and below the aneurysmal bulge into the nondilated, if possible healthy aorta and iliac artery. The proximal anchorage is the so-called “neck of the aneurysm.” Compared with open surgery, the endovascular procedure is not only less invasive but also hemodynamically better tolerated because transient clamping of the subrenal aorta is not necessary. This technique of endovascular AAA repair using stent-graft prostheses was introduced into clinical practice following a series of experimental studies conducted independently by Volodos and Parodi in the late 1980s and early 1990s.14,44,45
Morphology of the Abdominal Aortic Aneurysm
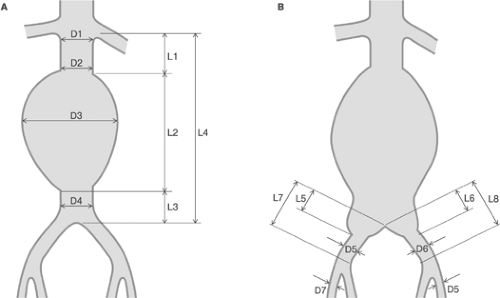
The basic requirement for EVAR is suitable morphology of the AAA and iliac arteries allowing safe introduction and reliable expansion and anchoring of the stent graft. However, it should be noted that the morphologic criteria of EVAR change with time, corresponding to the evolution of endovascular techniques and technology. The morphology of AAA and iliac arteries is evaluated on the basis of imaging and the measurement of selected parameters of the infrarenal aorta with the aneurysm and iliac arteries (Fig. 10-1). In planning EVAR, the most critical part of the abdominal aorta is the region between the take off of the renal arteries and the bulge of the aneurysm—the so-called proximal neck of the aneurysm. The diameter, length, and shape of the proximal neck along with the presence of calcifications and thrombi are evaluated to determine the suitability of an individual pathoanatomic substrate for EVAR. The pathoanatomic quality of the proximal neck is also important for the long-term outcome and stability of the implanted stent graft. Furthermore, the aneurysmal sack and the region of
bifurcation are measured to determine the diameter and length of the aneurysmal sack and evaluated to determine the presence of thrombus within the aneurysmal sack. An equally important parameter is the angle of the longitudinal axis of the aneurysm and the longitudinal axis of the aneurysmal neck, which should be <60 degrees. The availability of a distal neck, that is, a healthy section of the aorta below the aneurysm, determines the selection of the type of stent graft. In AAA without a distal neck, the diameter of the bifurcation determines whether sufficient space is available to pass through both sleeves of a bifurcation-type stent graft, which is appropriate for a given location. If the aneurysm extends distal to the bifurcation, the extent of the involvement of the iliac arteries is examined. The aneurysmal sack is further evaluated for the presence and number of patent vessels that arise from aneurysmal sack (i.e., lumbar arteries, inferior mesenteric artery, accessory renal arteries, internal iliac arteries). Depending on their number, these vessels may become the source of a clinically relevant retrograde flow into the aneurysmal sack, thus compromising the outcome.46 The iliac arteries are evaluated with regard to dimensions (length and diameter), shape (tortuosity), and arterial wall quality (stenosis, patency, ulcerations, calcifications, mural thrombosis). In summary, the most critical parameters in determining the technical feasibility of EVAR include the length of the proximal neck and the degree of axial deviation of the infrarenal aorta. In standard procedures, that is, not considering the new and thus far experimental technical options (e.g., fenestrated stent graft, branched stent graft) or combined endovascular-surgical methods, the minimal length of the proximal neck should be 15 mm and the axial deviation of the infrarenal aorta <60 degrees.
bifurcation are measured to determine the diameter and length of the aneurysmal sack and evaluated to determine the presence of thrombus within the aneurysmal sack. An equally important parameter is the angle of the longitudinal axis of the aneurysm and the longitudinal axis of the aneurysmal neck, which should be <60 degrees. The availability of a distal neck, that is, a healthy section of the aorta below the aneurysm, determines the selection of the type of stent graft. In AAA without a distal neck, the diameter of the bifurcation determines whether sufficient space is available to pass through both sleeves of a bifurcation-type stent graft, which is appropriate for a given location. If the aneurysm extends distal to the bifurcation, the extent of the involvement of the iliac arteries is examined. The aneurysmal sack is further evaluated for the presence and number of patent vessels that arise from aneurysmal sack (i.e., lumbar arteries, inferior mesenteric artery, accessory renal arteries, internal iliac arteries). Depending on their number, these vessels may become the source of a clinically relevant retrograde flow into the aneurysmal sack, thus compromising the outcome.46 The iliac arteries are evaluated with regard to dimensions (length and diameter), shape (tortuosity), and arterial wall quality (stenosis, patency, ulcerations, calcifications, mural thrombosis). In summary, the most critical parameters in determining the technical feasibility of EVAR include the length of the proximal neck and the degree of axial deviation of the infrarenal aorta. In standard procedures, that is, not considering the new and thus far experimental technical options (e.g., fenestrated stent graft, branched stent graft) or combined endovascular-surgical methods, the minimal length of the proximal neck should be 15 mm and the axial deviation of the infrarenal aorta <60 degrees.
At present the morphology of the aneurysmal sack is determined and all EVAR-related measurements are preferably performed using computed tomography angiography (CTA). Although thin-cut (<3 mm) conventional dynamic CT scanning may be adequate, helical/spiral scanning with multiplanar reconstruction is preferable.47 The measurement of the diameter must be performed at right angles to the longitudinal axis of the artery. The longitudinal measurements are done while taking into consideration the expected course of the stent graft within the aneurysm so as to exclude possible inaccuracies.48 Alternatively, but less commonly, the preinterventional assessment of the AAA may be performed using abdominal aortic digital subtraction angiography (DSA) with a calibrated catheter in place to determine the length of the aneurysm and iliac arteries for selecting the optimum stent graft prosthesis for each individual patient (Tables 10-1 and 10-2).47 The advantage of DSA is the exact definition of blood supply and vascular networks of the visceral organs, spine, and pelvis.
To improve treatment decisions, a morphological classification of AAA was introduced that is based on the evaluation of the extent of the aneurysm with respect to the presence and length of the upper and lower aortic neck. Systematic use of the classification allows better matching and selection of patients for EVAR and the specific types of stent grafts, and permits standard comparisons of outcomes to be made on the basis of
the anatomic substrate. Schumacher and EUROSTAR morphological classifications are currently employed (Fig. 10-2).49,50
the anatomic substrate. Schumacher and EUROSTAR morphological classifications are currently employed (Fig. 10-2).49,50
TABLE 10-1. Targets of Preinterventional Diagnostic Evaluations in Patients with Abdominal Aortic Aneurysm (AAA) | ||
|---|---|---|
|
TABLE 10-2. Suggested Diagnostic Techniques to Evaluate Patients with Abdominal Aortic Aneurysm Considered for Endovascular Treatment | ||
|---|---|---|
|