Abdominal Aorta, Iliac, and Lower Extremity Arteries
Peter Lanzer
Ralf Weser
Peripheral Arterial Disease
The term peripheral arterial disease (PAD) means different things to different people. For example, in the recent joint ACC/AHA publication, PAD definition included the infradiaphragmatic arteries, i.e., abdominal aorta, renal arteries, mesenteric arteries, and arteries of the lower extremities.1 In the context of this textbook peripheral arterial disease (PAD) comprises diseases of the distal abdominal aorta, iliac (aortoiliac segment), and infrainguinal arteries, divided into a femoropopliteal and infrapopliteal segment. Arterial diseases of the upper arm have not been included primarily because in that vascular bed percutaneous revascularization is rarely applied and, if needed, it follows similar principles to those presented. Because of the continuity of peripheral vessels from proximal to distal, disease of the proximal vessels always affects all downstream vessels (run-in effect). Conversely, any disease of the downstream vessels adversely affects the upstream circulation (runoff effect). Therefore, from the pathophysiological standpoint, vessels distal to the distal abdominal aorta form a complex functional unity of interacting vascular segments. However, for the purposes of endovascular therapy, separation of the peripheral vascular bed into vascular segments and territories is useful, mainly because of differences in vessel structure and morphology that prescribe different interventional strategies. Figure 12-1 shows the anatomic and functional continuity of the peripheral arterial vascular bed and its endovascular partition.
PAD is caused in the vast majority of patients by atherosclerosis and diabetic vasculopathy.2 Other causes, including vasculitides3 and obliterating thrombangitis of Winiwarter and Buerger,4 are rare by comparison. In specific vascular beds other etiologies must be considered in differential diagnostics. For example, in the popliteal artery, an entrapment syndrome may occur due to outside compression of the artery by the gastrocnemius, popliteus, or soleus muscles,5,6 as may aneurysms7 and cystic adventitial disease (compression of the artery by mucoid adventitia-derived cysts).8,9 In all cases of suggested PAD, nonvascular causes of symptoms (pseudoclaudication) such as nerve root compression, spinal stenosis, hip arthritis, and others must be considered and excluded.10
Atherosclerotic PAD may be one of the most frequently undetected, chronic, debilitating disorders, and it is certainly the most frequently unrecognized atherosclerotic cardiovascular disease (CVD). This is surprising given the availability of a highly sensitive (95%) and highly specific (100%) screening test, namely, the ankle-brachial index (ABI).11
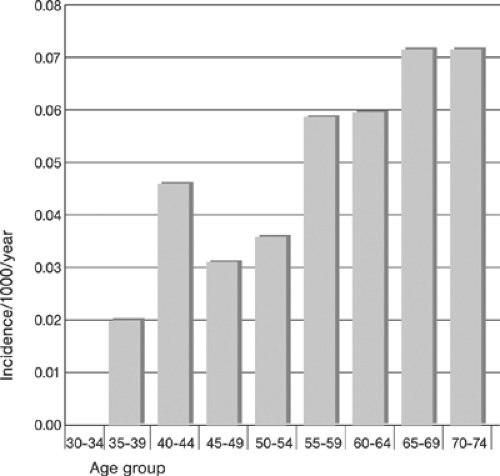
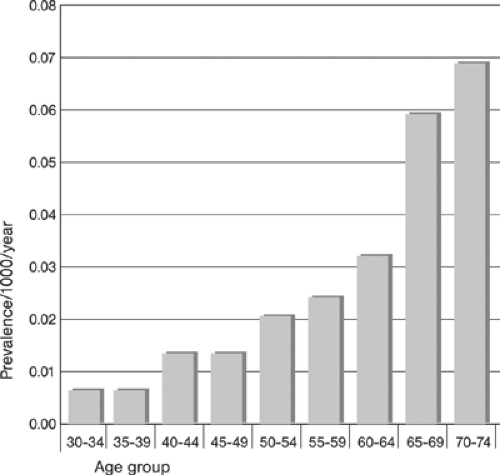
Depending on the population studied and the method of detection utilized, the prevalence and the incidence of PAD reported in the literature vary widely. Table 12-1 provides an example of prevalence reported in individual studies12; Figures 12-2 and 12-3 show the summary incidence and prevalence from large population-based studies.13 Using objective criteria for evidence of PAD (ABI measurements) and CVD, defined as a history of atherosclerotic coronary, cerebral, and abdominal aortic aneurysmal disease in a selected population of patients (>70 years and 50 to 69 years with diabetes and/or cigarette smoking ≥10 pack years), the overall prevalence of PAD was 29%! The prevalence of isolated PAD was 13%; that of PAD associated with CVD was 16%. Newly detected, that is, previously unrecognized, PAD was present in 13% of patients (7% in the
isolated PAD group and 6% in the combined PAD and CVD group), suggesting that in this selected population, 45% of the patients with PAD were not aware of its presence! 14
isolated PAD group and 6% in the combined PAD and CVD group), suggesting that in this selected population, 45% of the patients with PAD were not aware of its presence! 14
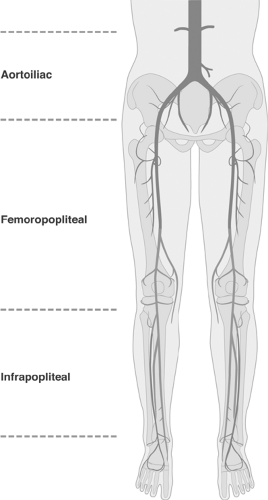 FIGURE 12-1. Anatomic and functional continuity of the peripheral arterial vascular bed and its endovascular partition into aortoiliac, femoropopliteal, and infrapopliteal segments. |
TABLE 12-1. Prevalence of Peripheral Arterial Disease, Claudication, and Concomitant Cardiovascular Disease in Selected Studies | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
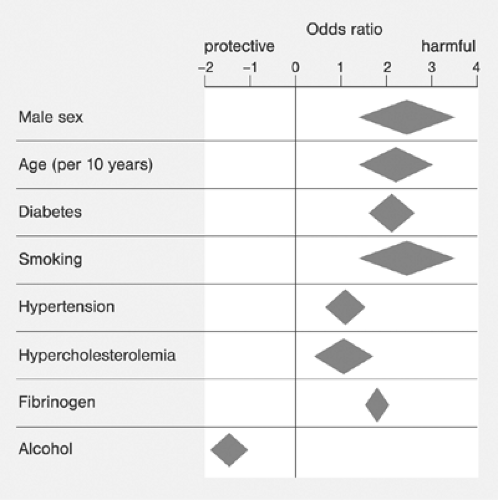
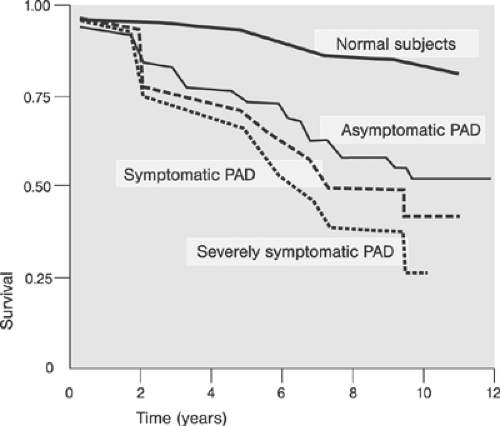
Major modifiable risk factors for PAD include diabetes and cigarette smoking Fig. 12-4. An example of the incidence of cardiovascular risk factors in individuals with and without PAD is shown in Table 12-2.15 Critical prognosis of PAD, primarily as a harbinger of CVD morbidity and mortality has been well documented16 and widely recognized.11 Figure 12-5 compares the 10-year survival of patients with and without PAD.
The pace of clinical progression of PAD remains a matter of debate; earlier reports suggested a rather benign course17,18 with approximately 25% of patients progressing from claudication to intervention (for review, see reference 13), but in a more recent study, marked progression was noted in individuals with ABI <0.50.19 Risk factors of progression appear identical to those of PAD (for review, see reference 13).
Depending on the severity of the lower leg ischemia, PAD may be associated with the clinical symptom of intermittent claudication (buttocks, hips, thighs, calves, and feet) or in more advanced cases with rest pain. However, in a large number of patients, PAD may remain asymptomatic or it may present with nonspecific and atypical symptoms. To stress the critical importance of revascularization in patients with advanced PAD associated with threat to the limb, the syndromes of chronic and chronic critical or acute lower extremity ischemia have been introduced as specific entities. To classify the patient properly thorough history including documentary as to the nature, duration, and temporal course of symptoms is absolutely critical.
On the basis of clinical symptoms, the Fontaine classification20 in modified form (replacing stage IV for stages IVa and IVb and introducing stages IIa and IIb)21 distinguishes four different stages of severity of chronic leg ischemia (Table 12-3). A similar classification of chronic leg ischemia based on clinical findings and blood pressure measurements at rest and during exercise, has been proposed by Rutherford et al. (Table 12-4) and implemented in clinics.22,23,24,25
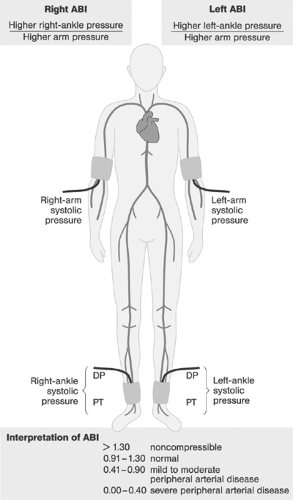
Besides history and physical examination, ABI represents the single most important test in diagnosing PAD. Figure 12-6 shows the definition of points of ABI measurement and the interpretation of findings. In patients with peripheral artery calcifications (ABI >1.1), toe blood pressure and toe/brachial index may be used to distinguish between PAD and nonobstructive arterial wall hardening.26
Noninvasive diagnostic techniques applicable to the assessment of PAD have been reviewed in the literature.1,27,28 Magnetic resonance angiography (MRA) of peripheral arteries provides a
useful road map for targeting endovascular interventions29 and may become an important adjunct in specific settings.30 However, in peri-interventional settings, digital subtraction angiography (DSA) continues to be uniquely positioned to provide a road map and to guide peripheral catheter-based interventions.
useful road map for targeting endovascular interventions29 and may become an important adjunct in specific settings.30 However, in peri-interventional settings, digital subtraction angiography (DSA) continues to be uniquely positioned to provide a road map and to guide peripheral catheter-based interventions.
TABLE 12-2. Cardiovascular Disease Risk Factors among Persons with and without Prevalent Peripheral Arterial Disease (PAD) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 12-3. Intermittent Claudication, Modified Fontaine Classification | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 12-4. Clinical Categories of Chronic Lower Limb Ischemia According to Rutherford et al. | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Critical Limb Ischemia
The term critical ischemia was originally introduced to designate ischemia requiring successful revascularization to avoid amputation.31 The originally proposed objective criteria to define critical ischemia (ankle pressure <40 mm Hg; resting pain and <60 mm Hg; ulceration) were later modified, and the term chronic critical limb ischemia (CLI) has been coined to denote a condition with “chronic ischemic rest pain, ulcers, or gangrene attributable to objectively proven arterial occlusive disease.” Furthermore the “term critical limb ischemia implies chronicity and is to be distinguished from acute limb ischemia. In most cases a major amputation would be expected within the next 6 months to a year in the absence of a significant hemodynamic improvement” (for review, see reference 13). Table 12-5 summarizes the proposed objective criteria required for establishing the diagnosis of CLI.
Patients presenting with CLI typically have rest pain in the affected limb requiring narcotic medication for analgesia. The pain may improve with dependency and worsens if the patient is in the supine position. The immediate objectives of the diagnostic evaluations include confirmation of the diagnosis and precise anatomic definition of the status of the frequently multilevel PAD allowing definite decisions as to the feasibility, timing, and strategy of revascularization.
The choice of diagnostic modalities and interim management options depends on a number of factors including presence of coexistent morbidities, status of the cardiac and renal function, and other factors, and this choice must be tailored to the needs of individual patients. In all cases, a thorough risk and benefit analysis by a team consisting of vascular specialists, interventionists, and vascular surgeons is required.
TABLE 12-5. Suggested Criteria of Chronic Critical Limb Ischemia by TransAtlantic Inter-Society Consensus (TASC) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
TABLE 12-6. Reversibility of Ischemic Injury in Patients Presenting with Acute Limb Ischemia (ALI) | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
Acute Limb Ischemia
Acute limb ischemia (ALI) has been defined as “any sudden decrease or worsening in limb perfusion causing a potential threat to extremity viability.”13 More recently ALI was defined as “any sudden decrease in or worsening of limb perfusion causing a threat to extremity mobility and viability that has been present for less than 14 days.”32 Although ALI due to acute thrombosis or embolization, often without a critical PAD, and CLI often due to decompensation of advanced PAD, sometimes associated with thrombotic occlusions, represent distinct pathogenetic entities in clinical practice, their differentiation can be difficult. A detailed history as to the timing and time course of the symptoms and clinical and laboratory examinations focusing on the detection of the presence and severity of the underlying PAD usually will provide clues to allow the distinction. To plan treatment, most importantly a definite anatomic definition of the involved vascular bed including the status of the run-in and runoff vessels and the status of the extremity with regard to the severity of ischemia, potential reversibility, threat to viability, and risk of ischemia-reperfusion injury must be established. However, the frequently protracted course and great variability of ischemic tolerance of the peripheral skeletal muscle in individual patients make the assessment of viability and prognostication of reversibility and reperfusion injury difficult, occasionally even for experienced physicians with access to a complete vascular diagnostic battery. Table 12-6 summarizes some of the findings associated with ischemic injury in patients presenting with ALI.
Diagnostic and Peri-Interventional Aortoiliac and Peripheral Arteriography
With the advances made in noninvasive computed tomography (CT) and magnetic resonance (MR) peripheral angiography and its increasing ability to localize and to define disease,33,34 the indications for diagnostic x-ray digital subtraction angiography (DSA) have now become limited to specific settings, frequently depending on the actual quality and validity of noninvasive studies in individual patients. To allow definitive decisions on the need for and technical feasibility of peripheral arterial revascularization, diagnostic arteriography should provide a full anatomic and morphologic definition of arteries and lesions from the infrarenal abdominal aorta down to the arteries of the feet. Depending on the findings such as presence of stenoses, status of collateral vessels, and vascular anomalies, additional series of specific vascular regions might be required. In cases where the noninvasive diagnostic MR or CT angiography provides all required information, x-ray angiography is used only during the intervention. In cases with suboptimal MR or CT image quality or incomplete vascular definition, diagnostic x-ray angiography is prescribed to complete the study and to allow definitive statements. In patients with complex multilevel PAD, diagnostic x-ray angiography might be preferable, allowing a single-stage definitive assessment and treatment decisions. Peripheral DSA studies may be performed using either automated preprogrammed series of DSA images timed to the progress of the contrast agent downstream in the peripheral arteries (bolus-chase peripheral DSA angiography) or static DSA with manual adjustment of every subsequent imaging level. Since image quality is better when static masks are used for subtraction35 and because of frequent disparities between the speed of travel of the contrast agent between the left and right leg, static DSA peripheral arteriography is preferable in most cases.
To perform a full diagnostic evaluation of the peripheral vasculature using DSA, the patient is securely positioned on the table with his legs brought together, resting quietly and fastened to avoid motion. When a standard femoral or brachial access has been established, a pigtail catheter is positioned proximally (suprarenal aortography) or distally (infrarenal aortography) to the take-off of the renal arteries and sequential DSA series are acquired using preprogrammed set of image acquisition parameters, spanning the entire region of interest in anteroposterior projection. Examples of standard imaging protocols are provided in Table 12-7.36 Adjustments of individual imaging parameters might be required to account for variable regional pathoanatomy and hemodynamics. To improve the distal peripheral inflow after the acquisition of abdominal aortic angiograms, the pigtail catheter is repositioned just above the aortic bifurcation to acquire peripheral runoff angiograms. In patients with disparate velocity of blood flow between the two legs and complex multilevel disease, side-selective or additional segmental peripheral angiograms might be needed to fully define the peripheral arterial pathoanatomy. In patients with a severe distal disease, additional DSA series via antegrade fine-needle or small-size sheath (3F) access might be required.
TABLE 12-7. Examples of Standard Imaging Protocols for Peripheral Digital Subtraction Angiography (DSA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
To open up the left common iliac artery and the right common femoral artery bifurcations, an additional right anterior oblique projection acquired at the appropriate level may be useful, as is the case with an additional left anterior oblique projection to define the bifurcations of the right common iliac artery and the left common femoral artery. To visualize stenotic lesions a second projection, preferably perpendicular to the anteroposterior (AP) projection (lateral projection), should be acquired. During the imaging at the ankle level, the feet should be rotated externally to allow a better separation of the three infrapopliteal vessels. To improve visualization of the distal runoff vessels, reactive hyperemia following inflation of the blood pressure cuff at the thigh (20 mm Hg above systolic pressure for 3 to 5 minutes) or intra-arterial administration of a vasoactive agent such as calcium channel blockers (e.g., diltiazem 5 mg) might be helpful. However, the patient’s tolerance of ischemic pain associated with blood pressure cuff inflation might limit the clinical applicability of this approach. In patients with severe obstructions of the pelvic arteries or common femoral disease, preferably the left brachial access should be selected.
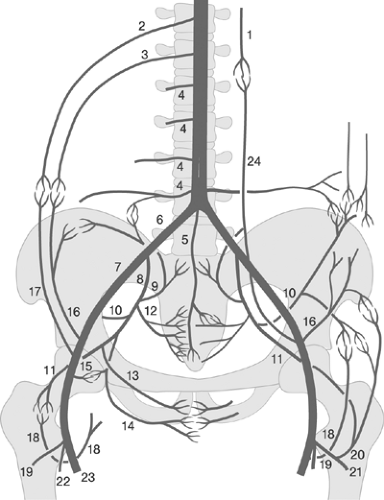
Exact knowledge of peripheral vascular anatomy is required for optimum image acquisition and interpretation. A simplified schema of the clinical vascular anatomy of the peripheral circulation is shown in Figure 12-7. In addition, the interventionist must know the principal peripheral collateral pathways and must recognize their functional significance in individual patients in order to understand the numerous potential steal and flow-reversal phenomena, particularly in cases considered for catheter-based and/or surgical revascularizations or those with previous peripheral artery bypass surgeries or interventions. Figure 12-8 shows the principal collateral pathways in the presence of occlusions in the aortoiliac and iliofemoral segments.37 Figure 12-9 demonstrates the principal collateral pathways of the femoropopliteal segment.38 Because of its importance as a natural collateral channel, the anatomy of the deep femoral artery and its branches should be clearly depicted and visualized. Taking off from the posterolateral border of the common femoral artery, usually 3 to 4 cm distal to the inguinal ligament, it usually provides medial and lateral branches supplying the adjacent muscles and the head of the femur, along with four perforating branches that perforate the dorsal muscle and are a rich source for intra- and interarterial collateralization between the common femoral and the popliteal arteries.
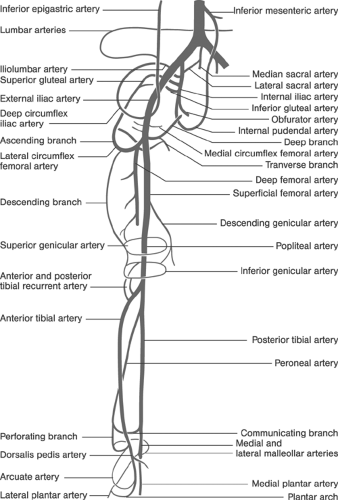
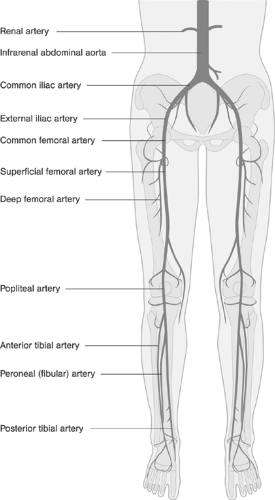 FIGURE 12-7. Clinical anatomy of the principal peripheral arteries relevant to the peripheral arterial interventions. |
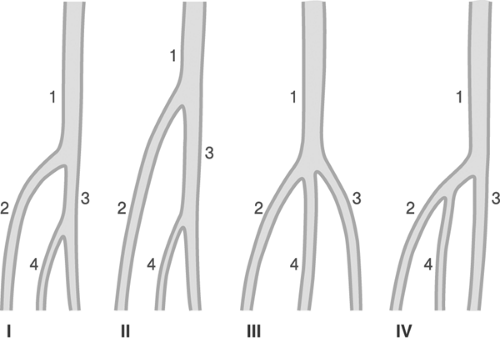
The popliteal artery extends from the inferior border of the adductor canal up to the take-off of the anterior tibial artery. Its course may be conveniently divided into three parts, proximal (part I) between the distal end of the adductor channel and the inlet into the tunnel of the gastrocnemius, middle (part II) up to the horizontal plane of the proximal border of the knee cleft, and distal (part III) up to the take-off of the anterior tibial artery, segments (Figure 12-10).39 Variations in the division of the popliteal artery are shown in Figure 12-11.
With increasing ability to treat infrapopliteal disease using a coronary-like approach to peripheral interventions, the functional anatomy of the three principal lower leg arteries, namely, the posterior and anterior tibial and fibular (peroneal) arteries, must also be clearly understood. The anterior tibial artery
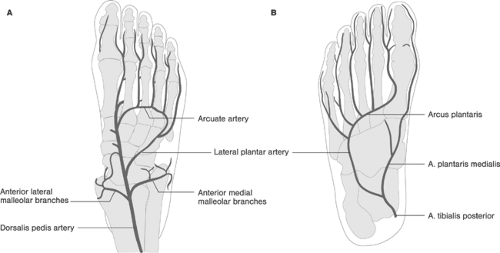
perforates the interosseus membrane between the tibia and fibula and courses distally between the extensor muscles of the lower leg to become the dorsalis pedis artery at the dorsum of the foot; this gives off the arcuate artery, which runs forming an arch to the level of the tarsometarsal line. The five dorsal metatarsal arteries arising from the arcuate artery continue as the dorsal digital arteries to the toes. The other terminal branch of the popliteal artery, the posterior tibial artery, descends straight toward the medial malleolus, while the peroneal (fibular) artery descends toward the lateral malleolus. The larger of the two terminal branches of the posterior tibial artery, the lateral plantar artery, forms the plantar arch at the base of the metatarsal bones; this in turn forms the plantar metatarsal arteries for the second, third, and fourth toes, becoming the common and proper plantar digital arteries. The lateral side of the fifth toe is usually supplied by an artery directly arising from the lateral plantar artery, whereas the first toe is largely supplied by the usually smaller medial plantar artery. Thus, the arterial blood supply to the feet is mainly provided by the arcuate artery of the anterior tibial (dorsal supply) and plantar arch of the posterior tibial (plantar supply). However, the peroneal artery might become an important supplier of collaterals to the feet in the presence of tibial artery obstructions. Figure 12-12 shows the anatomy of the arterial supply of the feet.
perforates the interosseus membrane between the tibia and fibula and courses distally between the extensor muscles of the lower leg to become the dorsalis pedis artery at the dorsum of the foot; this gives off the arcuate artery, which runs forming an arch to the level of the tarsometarsal line. The five dorsal metatarsal arteries arising from the arcuate artery continue as the dorsal digital arteries to the toes. The other terminal branch of the popliteal artery, the posterior tibial artery, descends straight toward the medial malleolus, while the peroneal (fibular) artery descends toward the lateral malleolus. The larger of the two terminal branches of the posterior tibial artery, the lateral plantar artery, forms the plantar arch at the base of the metatarsal bones; this in turn forms the plantar metatarsal arteries for the second, third, and fourth toes, becoming the common and proper plantar digital arteries. The lateral side of the fifth toe is usually supplied by an artery directly arising from the lateral plantar artery, whereas the first toe is largely supplied by the usually smaller medial plantar artery. Thus, the arterial blood supply to the feet is mainly provided by the arcuate artery of the anterior tibial (dorsal supply) and plantar arch of the posterior tibial (plantar supply). However, the peroneal artery might become an important supplier of collaterals to the feet in the presence of tibial artery obstructions. Figure 12-12 shows the anatomy of the arterial supply of the feet.
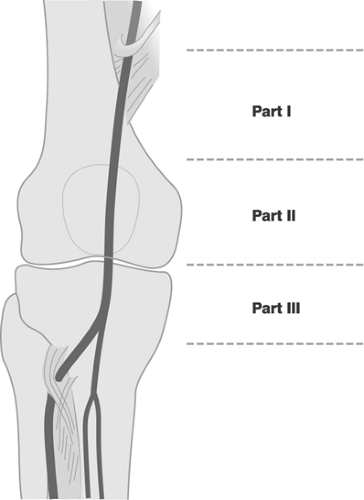 FIGURE 12-10. Division of the popliteal artery into segments, parts I-III. Redrawn from Diehm C, Allenberg J-R, Numura-Eckert K. Farbatlas der Gefäßkrankheiten. Berlin: Springer, 1999. |
Besides understanding normal vascular anatomy, including the most common vascular anomalies40 and pathoanatomy of atherothrombotic peripheral artery disease,38,41 specific findings relevant to percutaneous interventions should also be recognized. Thus, in patients with documented aneurysms and pseudoaneurysms, angiographic definition of the site—size (small to giant), form (saccular or berry; fusiform), and neck (width, spatial orientation)—is also required. In patients with documented abnormal connections between the arteries and
veins of the leg, an effort should be made to distinguish between the congenital and acquired forms, the latter being in most cases due to injuries associated with simultaneous penetration of neighboring arteries and veins. Arteriographic definition of arteriovenous fistulas includes definition of the feeding arteries and connecting channels as well as distal venous and arterial circulation, and bridging collateral circulation. Arteriographic definition of arteriovenous congenital malformations must provide a complete road map to allow treatment considerations if required (not considered in this chapter). The angiographic appearance of obliterating thrombangitis of Winiwarter and Buerger (a corkscrew appearance in the bridging collaterals and paucity of the typical signs of diffuse atherosclerosis) should be recognized (for review, see references 38, 41, and 42).
veins of the leg, an effort should be made to distinguish between the congenital and acquired forms, the latter being in most cases due to injuries associated with simultaneous penetration of neighboring arteries and veins. Arteriographic definition of arteriovenous fistulas includes definition of the feeding arteries and connecting channels as well as distal venous and arterial circulation, and bridging collateral circulation. Arteriographic definition of arteriovenous congenital malformations must provide a complete road map to allow treatment considerations if required (not considered in this chapter). The angiographic appearance of obliterating thrombangitis of Winiwarter and Buerger (a corkscrew appearance in the bridging collaterals and paucity of the typical signs of diffuse atherosclerosis) should be recognized (for review, see references 38, 41, and 42).
In patients with indeterminate or borderline angiographic lesions on standard angiograms, additional projections should be acquired. In addition, measurements of translesional pressure gradients may be helpful, although numerous cutoff values of systolic and mean pressure gradients measured at rest and after vasodilatation indicative of the hemodynamic significance of a stenotic lesion have been reported in the literature (for review, see references 43 and 44). Despite the fact that the most accurate measurements of translesional pressure gradients can be achieved using the flow/pressure-wire technology,45 in the clinical environment the majority of measurements are performed using the traditional fluid-filled systems. Here, diagnostic catheters or sheaths of various designs are applied employing various techniques such as simultaneous measurements using a single catheter with two ports, continuous measurements using a single catheter with one port during withdrawal, and simultaneous measurements obtained from the catheter tip and introductory sheath.43 According to the recommendations of the TransAtlantic Inter-Society Consensus (TASC), stenoses with resting mean pressure gradients greater than 5 to 7 mm Hg (bar) or postvasodilatation gradients greater than 10 to 15 mm Hg (bar) should be considered hemodynamically significant and indicative for revascularization.3
Because of the high percentage of interventions performed in patients with previous reconstructive vascular surgery, knowledge of the principal bypass channels is also required. Thus, the course and the proximal and distal anastomoses of the inter- and intrasegmental grafts—aortoiliac, iliofemoral, proximal and distal femoropopliteal, femorocrural, popliteocrural, crurocrural, axillofemoral, crossover femorofemoral, and other variants—and their functional importance for blood supply to the lower extremity must be clearly understood.
During procedures on the aortoiliac, femoropopliteal, and infrapopliteal (tibioperonal) arteries, only arterial DSA and fluoroscopy is sufficiently accurate to guide the interventions.
In DSA angiography-guided peripheral interventions, standard imaging protocols are implemented, consisting of selected projections of the interventional site and the distal runoff to document the baseline (preinterventional), intermediate (interventional), and final (postinterventional) findings. Pre- and postinterventional angiograms must include documentation of the peripheral runoff vessels. During the procedure, road mapping and fluoroscopy are used to guide the individual steps of the interventional cycle. Interventionists performing peripheral arterial interventions must be familiar with the angiographic representation of changing vessel wall morphology after interventions due to acute remodeling associated with plaque shifts, dissections, and perforations. Total fluoroscopy time and the amount of contrast agent used reflect on several variables including complexity of the disease, extent of the intervention, level of technical difficulty, and the quality of the procedure. Tables 12-8 and 12-9 provide examples of indications and relative contraindications for diagnostic peripheral arteriography.46 Table 12-10 provides an example of suggested complication thresholds for diagnostic peripheral arteriography.46
TABLE 12-8. Example of Suggested Indications for Diagnostic Peripheral Arteriography | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
TABLE 12-9. Example of Suggested Relative Contraindications for Diagnostic Peripheral Arteriography | |||||||
|---|---|---|---|---|---|---|---|
|
TABLE 12-10. Indicators and Thresholds for Complications in Diagnostic Peripheral Arteriography | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||
Peripheral Vascular Interventions: Basic Considerations
The first evidence for clinical potentials of endovascular interventions in femoropopliteal atherosclerotic disease was provided by Charles Dotter and Melvin Judkins, both radiologists at the Oregon Health Sciences University in Portland, Oregon, in their in 1964 published report.47 These interventions were performed using coaxial Teflon dilating catheters; later, double-lumen balloon catheters with a caged (“Korsett”) design were proposed,48 and finally low-compliance poly(vinyl chloride) balloons49 were implemented. In 1983, Dotter introduced the term stent for a coiled wire implant to scaffold a femoral artery in animal experiments50; the first peripheral stents for human use were introduced in the mid-1980s.51
Following the pioneering work of Charles Dotter, Andreas Grüntzig, and other “torch bearers,”52 peripheral vascular interventions were performed throughout the 1980s and early 1990s by interventional radiologists. The radiology-based approach to peripheral interventional procedures typically employed large-size sheaths (7-8F), 0.035-in. guidewires, and high-profile, over-the-wire (OTW) instrumentation. By contrast, the more recent coronary-like approach to peripheral interventions increasingly employed smaller systems (5F to 6F), 0.014-in. guidewires, and low-profile rapid exchange devices, all critically instrumental to the integrated coaxial telescopic interventional approach. Using the more facile and less traumatic instrumentation enabled broader indications for endovascular therapy and has led to proliferation and expansion of coronary and coronary-like techniques in noncoronary vascular territories including the renal, carotid, femoral, and infrapopliteal arterial interventions. At present these peripheral arterial interventions are performed by interventional radiologists, vascular surgeons, and, in rapidly increasing numbers, by interventional cardiologists. With increasing convergence of endovascular techniques, the new specialty of vascular (excluding coronary arteries) and panvascular (all vascular beds included) medicine has began to emerge. Because of the present heterogeneity of the field, the standards of practice and the criteria for training, performance, outcome, and quality assurance of peripheral interventions also vary among the involved professional societies. Selected issues relevant to the performance of peripheral interventions are briefly reviewed in the subsequent sections.
TABLE 12-11. Minimal Knowledge and Skills Required for Competence in Peripheral Catheter-based Interventions | |||
|---|---|---|---|
|
Training Requirements
Current recommendations for standard requirements include the joint statements of the American College of Cardiology, American College of Physicians, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society for Vascular Surgery (ACC/ACP/SCAI/SVMB/SVS) panel,53 and the older Society of Interventional Radiology (SIR) guidelines.54,55,56 Tables 12-11, 12-12, 12-13 present summaries of the requirements on competence in catheter-based peripheral vascular interventions proposed by the joint ACC/ACP/SCAI/SVMB/SVS panel. To achieve this broad competence in vascular interventions as proposed by the panel, centers of broad vascular or panvascular expertise will first need to be established. At present, only a very few selected centers would qualify to provide a fully comprehensive vascular or panvascular interventional training and education.
TABLE 12-13. Alternative Routes to Achieving Competence in Peripheral Catheter-based Intervention | ||||
|---|---|---|---|---|
|
Documentation and Reporting
On the basis of early reports,54,55 the need for the development of standards in documenting and reporting the outcomes of peripheral interventions has been recognized, and guidelines and recommendations that permit comparisons of data and comparison-based benchmarking have been successively developed.56,57 Although some of the definitions proposed in these documents may no longer apply, may be too cumbersome, or may be in need of revision, their essential parts are reproduced to illustrate these efforts, to encourage their adoption where applicable, or to stimulate their improvement where necessary. In Tables 12-14, 12-15, 12-16, 12-17, 12-18, the definition of patency, success, clinical improvement, complications, and length of follow-up periods are provided. In Tables 12-19, 12-20, 12-21, suggested standards for notes and reporting are reviewed, whereas Table 12-22 provides a suggested checklist for study protocols relevant to PAD.
TABLE 12-14. Definition of Patency in Peripheral Arterial Interventions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 12-15. Definition of Success in Peripheral Arterial Interventions. | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
Criteria for Indications
The criteria for indications for peripheral interventions are subject to change as progress is made in understanding the pathogenesis of the peripheral arterial disease and the perceived utility of competing and complementary treatments. In addition, the local expertise and availability of resources are decisive factors. In essence, however, the decisions to treat a patient with PAD by catheter-based interventions should be based on the
documentation of presumably greater benefits compared with medical or open surgery treatment. This documentation of presumably greater benefits should be based on objective evidence and local expertise required for successful treatment, and should result from a thorough risk and benefit analysis in a given patient.
documentation of presumably greater benefits compared with medical or open surgery treatment. This documentation of presumably greater benefits should be based on objective evidence and local expertise required for successful treatment, and should result from a thorough risk and benefit analysis in a given patient.
TABLE 12-16. Definition of Clinical Improvement in Peripheral Arterial Interventions | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
TABLE 12-17. Definition of Complications in Peripheral Arterial Interventions | ||||||||
|---|---|---|---|---|---|---|---|---|
|
TABLE 12-18. Suggested Definitions of the Length of Follow-up Periods | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
TABLE 12-19. Suggested Preprocedural Documentation for Peripheral Arterial Interventions. | ||||||||
|---|---|---|---|---|---|---|---|---|
|
TABLE 12-20. Suggested Immediate Procedure Note for Peripheral Arterial Interventions | ||||||
|---|---|---|---|---|---|---|
|
TABLE 12-21. Suggested Final Report for Peripheral Arterial Interventions | ||||||||
|---|---|---|---|---|---|---|---|---|
|
The decision on an optimum strategy may be relatively straightforward in “simple” cases, yet it may be difficult in complex ones. Similar to interventions in other vascular territories a thorough evaluation of the relevant patient-, PAD-, and target lesion-related
factors is critical to objective judgments. Critical decision criteria include presence of coexistent morbidities, in particular heart failure, renal dysfunction, and diabetes; vascular multimorbidity, in particular coronary and cerebrovascular disease; and severity and extent of the targeted PAD. In patients with PAD considered for catheter-based interventions, access to the target lesion, technical feasibility, and the expected long-term (!) outcome are also decisive. With the rapidly evolving coronary-like techniques applicable to infrainguinal interventions, and with better skills and instrumentation, the factors concerning technical feasibility are becoming less important, whereas considerations of clinical outcome, efficacy, costs, and available expertise are now decisive. Thus, although the primarily target lesion based criteria devised in the 1990s remain practical, their clinical validity and interpretation are increasingly subject to re-evaluation depending on the availability of locally available expertise and state of steadily improving peripheral endovascular instrumentation. Standard target lesion decision indicators are summarized in Table 12-23.58 However, rigid interpretations of these guidelines13,58 in clinical settings of aortoiliac, femoropopliteal, and infrapopliteal interventions appear no longer appropriate. Instead, experienced operators have learned to integrate this existing framework into far more comprehensive risk and benefit evaluations in individual patients. Indeed, in experienced centers increasing numbers of category 3 and 4 lesions (see Table 12-23) are now reasonable candidates for catheter-based therapy. To improve outcomes particularly in “complex cases,” that is, in patients with complex PAD and multiple coexisting morbidities,
interdisciplinary consensus decisions between interventionists and vascular surgeons and case-customized design of complementary revascularization strategies represent the best approach.
factors is critical to objective judgments. Critical decision criteria include presence of coexistent morbidities, in particular heart failure, renal dysfunction, and diabetes; vascular multimorbidity, in particular coronary and cerebrovascular disease; and severity and extent of the targeted PAD. In patients with PAD considered for catheter-based interventions, access to the target lesion, technical feasibility, and the expected long-term (!) outcome are also decisive. With the rapidly evolving coronary-like techniques applicable to infrainguinal interventions, and with better skills and instrumentation, the factors concerning technical feasibility are becoming less important, whereas considerations of clinical outcome, efficacy, costs, and available expertise are now decisive. Thus, although the primarily target lesion based criteria devised in the 1990s remain practical, their clinical validity and interpretation are increasingly subject to re-evaluation depending on the availability of locally available expertise and state of steadily improving peripheral endovascular instrumentation. Standard target lesion decision indicators are summarized in Table 12-23.58 However, rigid interpretations of these guidelines13,58 in clinical settings of aortoiliac, femoropopliteal, and infrapopliteal interventions appear no longer appropriate. Instead, experienced operators have learned to integrate this existing framework into far more comprehensive risk and benefit evaluations in individual patients. Indeed, in experienced centers increasing numbers of category 3 and 4 lesions (see Table 12-23) are now reasonable candidates for catheter-based therapy. To improve outcomes particularly in “complex cases,” that is, in patients with complex PAD and multiple coexisting morbidities,
interdisciplinary consensus decisions between interventionists and vascular surgeons and case-customized design of complementary revascularization strategies represent the best approach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree