HIV-infected patients have a greater prevalence of dyslipidemia, earlier incidence and progression of atherosclerosis, and a nearly twofold increased risk for myocardial infarction compared with those not infected with HIV. Pre-existing cardiovascular risk factors, viral replication, and antiviral treatments all contribute to this accelerated and increased risk for cardiovascular disease in HIV-infected subjects. Given this risk and the proven benefit of statins reducing cardiovascular events across numerous patient groups, statin therapy might be particularly beneficial for patients with HIV. However, safety concerns and a dearth of quality trial data evaluating clinical outcomes in HIV-infected patients on simultaneous antiretroviral therapy (ART) and statin therapy have likely limited statin use in HIV-infected patients chronically taking ART. We performed a systematic review evaluating 18 clinical trials of statins in HIV-infected subjects receiving ART. Simvastatin is contraindicated in the setting of protease inhibitor use because of toxic drug-drug interactions when the 2 drugs are taken concomitantly. Meanwhile, atorvastatin appears to be relatively safe at submaximal doses if monitored. Pravastatin, rosuvastatin, and pitavastatin appear to have the most benign safety profiles among statins when co-administered with ART and may not require dose adjustment. In conclusion, clinicians should be mindful of the elevated risk for atherosclerotic cardiovascular disease in HIV-infected patients when assessing the need for lifestyle interventions and statin therapy.
Antiretroviral therapy (ART) has dramatically improved survival of persons infected with HIV. As a result of their improved longevity and decreased likelihood of death due to AIDS-related illnesses, the expanding and aging population living with HIV is increasingly at risk for morbidity and mortality due to other non–AIDS-defining illnesses. Cardiovascular disease (CVD) occurs at an earlier age and more commonly in HIV-infected patients than in the uninfected population; HIV-infected adults have a 1.5- to 2-fold greater risk for myocardial infarction than those who are uninfected. Pre-existing CVD risk factors, HIV-specific risk factors, such as chronic inflammation and endothelial dysfunction, and side effects of some antiviral treatments appear to contribute to early and increased risk for atherosclerotic CVD (ASCVD) in patients with HIV infection. Statins are widely used in the treatment of dyslipidemia and prevention of ASCVD because of their ability to substantially reduce cardiovascular mortality—particularly because of myocardial infarction and stroke. Despite the prevalence of dyslipidemia in up to 80% of HIV-infected patients, relatively few—as low as 5.9% of HIV-infected patients on ART—are on statins. These relatively low rates of statin prescription may be because of concerns regarding statin metabolism and pharmacokinetics in the setting of concurrent ART. Table 1 outlines metabolism, pharmacokinetics, and potential significant ART drug-drug interactions of numerous statins.
| Drug | Metabolism | Bioavailability and Absorption (%s, respectively) | Lipophilic? | Potential Antiretroviral Interactions |
|---|---|---|---|---|
| Lovastatin | CYP3A4 | <5, 30 | Yes | PI, NNRTI |
| Simvastatin | CYP3A4, CYP3A4 | <5, 60-80 | Yes | PI, NNRTI |
| Pravastatin | Partial Hepatic (OATP1B1); Partial biliary/urinary excretion | 18, 24 | No | PI |
| Fluvastatin | CYP2C9, CYP3A4 (minor) | 25, 98 | Yes | |
| Atorvastatin | CYP3A4 | 12, 30 | Yes | PI |
| Rosuvastatin | CYP2C9 (<10%) | 20, rapid | No | PI |
| Pitavastatin | Glucuronidation, CYP2C9 | 51, 50 | Yes |
Methods
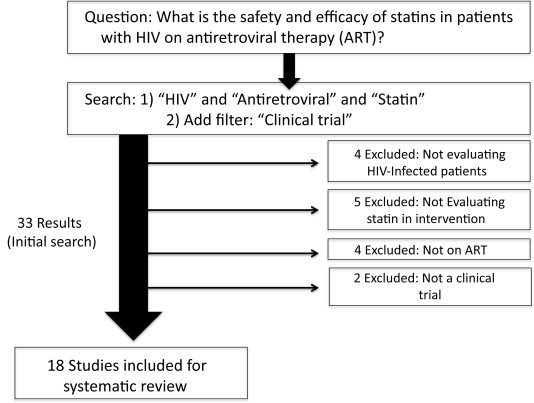
Prompted by concerns regarding adverse drug-drug interactions between ART and statins for HIV-infected patients, a number of clinical studies have evaluated safety and efficacy of statins for patients on ART. We conducted a systematic review of studies evaluating statin use in HIV-infected patients on ART. We focused on patients taking ART given their fundamental differences from the HIV population off ART with respect to lipid and metabolic profiles and concomitant co-morbidities, both of which significantly modify the impact of statins. Thus, we focused our search of statin studies to those performed in HIV populations on ART. Our central study question was: “What is the safety and efficacy of statins in patients with HIV on antiretroviral therapy?” To answer this question, we performed an advanced search on PubMed using the following search strategy: “HIV” and “Antiretroviral” and “Statin” with the filter for “clinical trial.” To ensure the studies under review related to the central study question, we decided a priori to exclude studies from these 33 if the studies did not evaluate HIV-infected patients on ART and a statin in the intervention or were not clinical trials. The results of this search strategy are shown in Figure 1 . Summaries of each trial are listed in Table 2 . Overall, we evaluated 18 clinical trials addressing statin use in HIV-infected subjects receiving ART. The size of the study samples ranged from only 12 to 301 participants, and there was substantial heterogeneity in the clinical characteristics, drugs tested, and outcomes ascertained in the patient groups studied. Accordingly, we undertook a narrative review of the findings of these 18 trials with regard to statin safety and efficacy rather than a formal quantitative meta-analysis.
| First Author | N | Year | Population | ART Type | Intervention (R=Randomized; NR=Non-Randomized) | Follow-Up (Wks) | Central Findings |
|---|---|---|---|---|---|---|---|
| Moyle | 31 | 2002 | Viral load < 500 copies/ml Total chol >240 mg/dl | PI | R: Diet/Pravastatin vs Diet | 24 | No impact of pravastatin on PI levels |
| Benesic | 25 | 2004 | Refractory hyperlipidemia | Any | NR: Pravastatin or Fluvastatin | 12 | Fluvastatin reduced LDL 30% Pravatatin reduced LDL 14% |
| Aberg | 174 | 2005 | Combined dyslipidemia (LDL≥130, TG ≥200) | Any | R: Pravastatin vs. fenofibrate | 48 | Pravatatin reduced LDL 30mg/dl No serious adverse events |
| Calza | 135 | 2005 | Mixed hyperlipidemia On first PI-based regimen | PI | R: NNRTI instead of PI vs. pravastatin with PI | 52 | Pravatatin/PI lowers LDL more than substituting NNRTI alone |
| Moncunill | 12 | 2005 | Viral load >1000 Discontinued ART | – | NR: Simvastatin | 12 | Simvastatin lowers LDL but does not change HIV viremia |
| Coll | 20 | 2006 | Elevated LDL | Any | R: Fluvastatin vs. Ezetimibe | 12 | Fluvastatin (-24%) and ezetimibe (-20%) decrease LDL |
| Hurlimann | 29 | 2006 | Hyperlipidemia | PI | R: Pravastatin vs. placebo | 8 | Pravastatin decreased LDL and improved endothelial function |
| Mallon | 33 | 2006 | Hyperlipidemia | PI | R: Pravastatin vs. placebo | 16 | No significant difference in change in total cholesterol |
| Manfredi | 301 | 2006 | Hyperlipidemia On ART ≥12 months | Any | NR: Pravastatin vs. fibrates vs. diet/exercise | 104 | Statins/fibrates do not significantly decrease CD4 |
| Negredo | 41 | 2006 | On ART | Any | R: ART interruption → atorvastatin vs. placebo | 12 | Atorvastatin does not reduce viral rebound after ART stopped |
| Soler | 32 | 2006 | Dyslipidemia due to ART | Any | NR: Atorvastatin and diet | 26 | Atorvastatin safe and efficacious for ART-related dyslipidemia |
| Calza | 94 | 2008 | Total cholesterol >250 despite diet/exercise | PI | R: Rosuvastatin vs. pravastatin, atorvastatin | 52 | Rosuvastatin most efficacious All three statins well-tolerated |
| Aslangul | 88 | 2010 | Dyslipidemia | PI | R: Rosuvastatin vs. pravastatin | 6.5 | Rosuvastatin (-37%) lowers LDL more than pravastatin (-19%) |
| Calmy | 45 | 2010 | Lipoatrophic patients on ritonavir-boosted lopinavir | PI | R: Uridine vs. pravastatin | 24 | Neither uridine nor pravastatin reduced limb fat mass |
| Fichtenbaum | 74 | 2010 | Combined dyslipidemia | Any | R: Pravastatin vs. fenofibrate | 48 | Pravastatin and fenofibrate reduce ApoB but not hs-CRP |
| Baker | 37 | 2012 | ≥3% 10 yr FRS, no other statin or ACE-i indication | Any | R (2×2): Lisinopril vs. pravastatin vs. placebo | 16 | Prava did not significantly reduce lipids or biomarkers but was safe |
| Calza | 36 | 2013 | Hypercholesterolemia and carotid atherosclerosis | Any | NR: Rosuvastatin | 104 | Rosuvastatin decreased mean carotid-IMT, was well-tolerated |
| Funderburg | 147 | 2015 | LDL ≤130 mg/dl Elevated Lp-PLA2 | Any | R: Rosuvastatin vs. placebo | 48 | Rosuvastatin decreased Lp-PLA2 |
Results
Given concerns regarding drug-drug interactions between ART (particularly protease inhibitor [PI] containing) regimens and statins, a number of studies have assessed the pharmacology and clinical safety profiles of statins when co-administered with ART. In a 2002 trial in which 31 HIV-infected patients with viral suppression on PI therapy were randomized to pravastatin 40 mg daily plus dietary advice versus dietary advice alone and followed for 24 weeks, the authors found no consistent or significant impact of pravastatin on PI levels. Similarly, a nonrandomized open-label trial in which 25 hyperlipidemic HIV-infected patients on ART including indinavir (an older PI no longer in routine use) for >2 years were given fluvastatin or pravastatin and followed for 12 weeks, neither statin was found to significantly influence plasma levels of indinavir. A larger trial in which 174 HIV-infected patients on ART were randomized to pravastatin 40 mg daily or fenofibrate 200 mg daily (with the potential for dual therapy after 12 weeks), collected safety data on patients for 48 weeks, and found a total of 1 pravastatin-related adverse reaction which was not considered serious. Similarly, a 2006 trial in which HIV-infected patients with hypercholesterolemia attributed to ART were given atorvastatin 10 or 20 mg daily demonstrated that atorvastatin was safe and well tolerated at these doses. Rosuvastatin also appears to be safe in HIV-infected patients; in a 2008 trial, 94 hyperlipidemic HIV-infected adults on stable PI-based ART for ≥12 months were randomized to rosuvastatin 10 mg daily, atorvastatin 10 mg daily, or pravastatin 20 mg daily, and all 3 statins demonstrated favorable tolerability profiles. Safety data from these trials are corroborated by data from a large retrospective cohort study of HIV-infected patients and uninfected controls that found no significant excess of clinical adverse events in HIV-infected patients taking statins.
In addition to their generally benign safety profiles in HIV-infected patients in these trials, statins appear to be efficacious in treating blood cholesterol in this population. In a nonrandomized study of 25 hyperlipidemic HIV-infected patients on long-term ART, low-density lipoprotein (LDL) cholesterol decreased by 30% to 35% in participants taking fluvastatin 20 to 40 mg daily and by 12% to 15% in patients taking pravastatin 10 to 20 mg daily. Fluvastatin also effectively lowered LDL (by a mean of 24% from baseline) in a separate study of 20 hyperlipidemic HIV-infected patients randomized to fluvastatin or ezetimibe. In a larger study of 174 HIV-infected patients randomized to pravastatin 40 mg daily versus fenofibrate, pravastatin reduced LDL from baseline by a mean of 30 mg/dl (a 20% reduction), which was a similar reduction as that seen in HIV-uninfected patients at a similar dose. Furthermore, a 2005 study of 135 HIV-infected patients on PI-containing ART with mixed hyperlipidemia found that pravastatin was significantly more effective in managing PI-related hyperlipidemia than simply substituting an non-nucleoside reverse transcriptase inhibitor (NNRTI) for a PI without adding lipid-lowering therapy. Atorvastatain and rosuvastatin have also demonstrated efficacy in lowering LDL cholesterol in the HIV-infected population; as occurring in the HIV-uninfected population, rosuvastatin appears to be somewhat more potent than atorvastatin and substantially more potent that pravastatin with regard to lowering LDL in HIV-infected patients. In a trial in which HIV-infected patients on PIs were randomized to atorvastatin 10 mg, rosuvastatin 10 mg, or pravastatin 20 mg, the mean reductions in LDL at 1 year were 20%, 25%, and 18% with these doses of atorvastatin, rosuvastatin, and pravastatin, respectively.
Of note, not all studies have demonstrated significant reductions in lipid parameters with statin use, although much of this may have been limited by study design. A 2006 trial of 33 hyperlipidemic HIV-infected patients on PI-based therapy randomized to pravastatin 40 mg daily versus placebo found no significant between-group difference in time-weighted change in total cholesterol for >16 weeks. However, in this study, LDL cholesterol was not measured, and the outcome used (time-weighted change in total cholesterol) may have contributed to relatively wide confidence intervals and low power to detect significant between-group differences. Likewise, a 2012 study of 37 HIV-infected patients on ART randomized in 2 × 2 factorial design to lisinopril 10 mg daily, pravastatin 20 mg daily, and/or matching placebo demonstrated no significant differences in lipid parameters for pravastatin versus placebo; however, this study was limited by a small sample size (10 or fewer patients per arm) and a suboptimal dose of pravastatin.
Statins also appear to be efficacious at reducing the burden of subclinical cardiovascular disease in HIV-infected patients. In a randomized, crossover, double-blind, placebo-controlled trial of 29 hyperlipidemic HIV-infected patients on PI-containing ART, pravastatin 40 mg compared with placebo improved endothelial function as measured by brachial flow–mediated dilation. Both pravastatin and fenofibrate significantly reduced ApoB and ApoB/A1 ratio in a study of 74 patients randomized to pravastatin or fenofibrate. Similarly, rosuvastatin 10 mg daily for 2 years was associated with a significant (20% to 25%) decrease in mean carotid intima-media thickness from baseline in a nonrandomized study of 36 HIV-infected patients on ART. In a randomized, double-blind, placebo-controlled study of 147 HIV-infected patients on stable ART with nonelevated LDL, rosuvastatin 10 mg significantly decreased Lp-PLA2 (a surrogate marker of inflammation and immune activation), sCD14, and IP-10 in comparison with placebo at 48 weeks. A randomized study of pravastatin 20 mg daily versus placebo in 37 HIV-infected patients did not demonstrate significant between-group differences in inflammatory biomarkers; however, the clinical relevance of this finding is unclear given the small sample size and low dose of pravastatin used. Finally, a randomized study in HIV-infected patients on ART evaluating pravastatin 40 mg daily versus placebo found no significant decrease in limb fat after 24 weeks of pravastatin therapy.
Although the statins described here appear to be relatively safe, efficacious at lipid lowering, and potentially beneficial with respect to subclinical CVD, the pleotropic effects of statins do not appear to modulate the HIV virus. In a small, single-arm study, 12 HIV-infected patients who interrupted ART for at least 12 weeks with viral rebound >1,000 copies/ml were administered simvastatin 80 mg daily for 12 weeks. There was no significant change in mean viremia or CD4 count on simvastatin. Likewise, a study that randomized 301 HIV-infected patients on ART to pravastatin, fibrates, or lifestyle intervention found no significant improvement in CD4 count with either statins or fibrates. Finally, in another trial, 41 HIV-infected patients interrupted ART and were randomized to atorvastatin 40 mg daily, atorvastatin 80 mg daily, or no additional medical therapy. Those randomized to atorvastatin (either dose) had no significant change in viremia or CD4 count compared with placebo.
In this systematic review, we included only studies that met the prespecified search criteria in an effort to minimize bias. However, this resulted in the exclusion of studies examining simvastatin and pitavastatin, 2 statins with very different safety profiles; simvastatin has greater toxicity when combined with PI-containing ART, whereas pitavastatin has a benign safety profile in the setting of HIV and ART. The primary underlying pharmacologic mechanism of these drug-drug interactions is thought to be dependence of some statins—particularly simvastatin, lovastatin, and to a lesser extent, atorvastatin—on cytochrome P450 3A4, which is intentionally inhibited by boosters in PI-containing regimens, resulting in not only improved pharmacokinetics of PIs but also increased systemic concentrations of other drugs metabolized by cytochrome P450 3A4. Our search of “HIV” and “Simvastatin” with the filter “clinical trial” revealed 5 studies, 2 of which were trials evaluating simvastatin that had not been within the primary 18 reviewed earlier. We subsequently attempted this strategy for pitavastatin (“HIV” and “Pitavastatin” with the filter “clinical trial”) although this yielded zero results. In a 2001 open-label study, atorvastatin 10 mg daily or simvastatin 20 mg daily were given to 32 HIV-uninfected subjects for 2 weeks and then nelfinavir (an older PI no longer in routine use) subsequently for 2 weeks. Nelfinavir dosing led to an increase in the steady-state and maximum concentrations of atorvastatin by 74% and 122%, respectively, and simvastatin by 505% and 517%, respectively. In light of these results, simvastatin is contraindicated in HIV-infected patients on PIs. The toxicity profile of simvastatin when co-administered with non–PI-containing ART regimens is less clear. A 2005 open-label study in which HIV-uninfected subjects were given simvastatin 40 mg, atorvastatin 10 mg, or pravastatin 40 mg daily and subsequently dosed with efavirenz (a commonly used NNRTI) demonstrated that efavirenz induced statin metabolism and decreased the concentrations of simvastatin, atorvastatin, and pravastatin by 58%, 34%, and 40%, respectively. Unlike simvastatin, pitavastatin has demonstrated a generally well-tolerated safety profile even when co-administered with PIs. A 2014 study in which HIV-uninfected subjects received pitavastatin 2 or 4 mg followed by either darunavir/ritonavir (a commonly used newer generation PI) or efavirenz demonstrated no significant pharmacokinetic interactions between pitavastatin and darunavir/ritonavir or efavirenz. Of note, ezetimibe has a distinct mechanism and pharmacokinetic profile from statins and appears to be relatively safe and efficacious with regard to lipid lowering in the HIV-infected population.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


