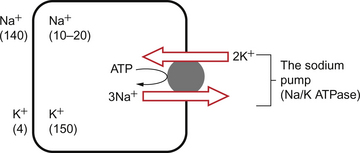
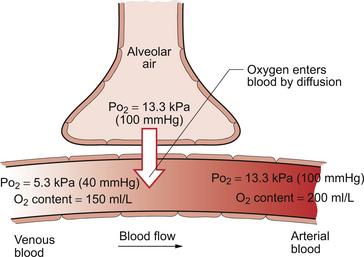
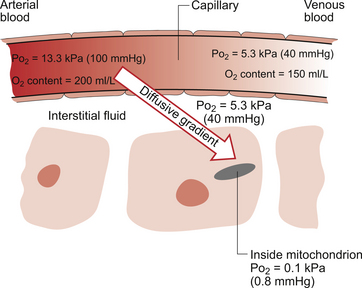
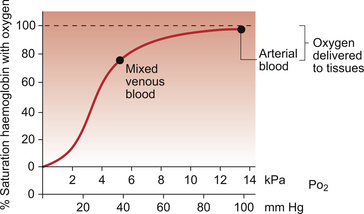
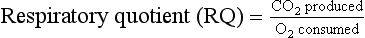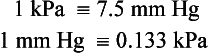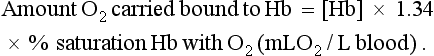1 As a consequence of these performance requirements we have evolved with a circulatory system which in the textbook person, if stretched out end to end, would measure 60 000 miles or 96 000 km. This is enough to encircle the world three times. This book is about the organization and control of this circulatory system, the causes and effects of failure and the basis for treatment regimens aimed at avoiding or minimizing the effects of circulatory failure. An example of a clinical history of a patient with developing circulatory problems is introduced in Case 1.1:1. Some fundamental concepts in relation to these opening paragraphs need further explanation. The precise value for RQ in any individual will reflect the composition of their diet but for a typical person consuming a mixed diet of carbohydrate, fat and protein the RQ would be about 0.8. This means that we normally consume more oxygen than we produce carbon dioxide. During exercise oxygen consumption may increase to about 10 times the resting value and the RQ may move closer to a value of 1 due to preferential metabolism of carbohydrate. Oxygen is used within the mitochondria of cells to generate adenosine triphosphate (ATP). This provides the energy for movement, for the synthesis of macromolecules and to drive the movement of ions, particularly Na+ ions, across cell membranes against a concentration gradient. The distribution of Na+ and K+ inside and outside cells is summarized in Figure 1.1. The ion gradients are maintained by the sodium pump which expels three Na+ ions and pulls two K+ ions into the cell each time it operates. As both of these ion movements are against a concentration gradient, an ATP molecule is hydrolysed to provide the energy. For some cells there may be about a million sodium pumps each operating at about thirty times a second. In the body as a whole the sodium pump accounts for about 30% of all of our energy intake over our lifetime. In this way, ionic gradients are maintained which are essential for the continuing function of nerves and muscles, including the heart. Failure to maintain ATP generation in hypoxic tissues leads to osmotic swelling of cells and to a loss of normal cellular function (see p.7). To serve all the requirements, the quantities of ATP which must be synthesized are quite prodigious and amount to something roughly equivalent to an individual’s body weight every day. Some important parameters determining rate of diffusion are: • the diffusive gradient (concentration difference between two points) • the solubility of the particle in the solvent; if it is not very soluble the concentration will be low • the size of the solute particle (small particles will diffuse faster than large particles) • temperature: diffusion is faster at high temperatures than at low temperatures; body temperature is about 37°C in normal human subjects. The diffusive gradients concerned with loading of oxygen into pulmonary capillary blood at the lungs and the delivery of oxygen into the tissues are shown in Figures 1.2 and 1.3. Figure 1.2 shows the events at the interface between an alveolus and a pulmonary capillary. A typical Po2 in the alveolus is 13.3 kPa (100 mm Hg). Blood returning to the lungs has a Po2 of about 5.3 kPa (40 mm Hg) and so oxygen diffuses into the pulmonary capillary blood from the alveolus. The diffusive gradient for unloading CO2 at the lungs is much smaller than for O2. Mixed venous blood Pco2 is about 6.1 kPa (46 mm Hg) whilst alveolar Pco2 is typically 5.3 kPa (40 mm Hg). The diffusive gradient for CO2 (0.8 kPa) is 10% of the diffusive gradient for O2 (8 kPa). Both diffuse at about the same rate because CO2 is 20 times as soluble in water as O2. The transit time for red blood cells through pulmonary capillaries at rest is about 1 second but the diffusive exchange of O2 and CO2 is normally complete in about 0.25 seconds. Delivery of O2 into the tissues (Fig. 1.3) starts with the arterial blood which has picked up O2 in the lungs (Po2 = 13.3 kPa; 100 mm Hg). Oxygen is used inside the mitochondria and the Po2 here is of the order of 0.1 kPa (about 1 mm Hg). The interstitial fluid outside a cell is part of the way down a continuous diffusive gradient between the arterial blood and the inside of a mitochondrion. A typical Po2 in the interstitial fluid is 5.3 kPa (40 mm Hg). Blood leaving a capillary has equilibrated with this fluid and so venous Po2 is the same as in the interstitial fluid. A further consequence of the poor solubility of oxygen in water is that we have evolved with an oxygen-carrying pigment, haemoglobin (Hb). The oxygen-binding characteristics of haemoglobin are such that it is nearly fully saturated with oxygen at the partial pressure of oxygen normally present in the alveoli of the lungs. Figure 1.4 shows the oxyhaemoglobin dissociation curve. At a Po2 of 13.3 kPa (100 mm Hg), a typical figure for the alveolus, Hb is 97–98% saturated with O2. This information can be used to calculate the amount of oxygen carried bound to haemoglobin as follows: Reference to the oxyhaemoglobin dissociation curve (Fig. 1.4) shows that venous blood is about 75% saturated with O2 at Po2 = 5.3 kPa (40 mm Hg) and therefore about one quarter of the O2 carried in arterial blood has moved into the tissues. One quarter of the 200 mL O2/L present in arterial blood is 50 mL. If 50 mL of O2 is typically deposited in the tissues from each litre of arterial blood, and the textbook person’s cardiac output (volume of blood pumped per minute from each side of the heart—see Chapter 4) is 5 L/min, then 250 mL O2/min is delivered to the tissues. This is the amount of oxygen identified previously as a figure for O2 consumption rate for the textbook person at rest. All tissues do not have the same oxygen consumption rate relative to blood flow. The figure quoted above, that ‘venous blood is typically 75% saturated with oxygen’, refers to ‘mixed venous blood’, i.e. the blood in the right side of the heart which is a mixture of all the venous drainages for the whole body. Venous blood from the kidneys, which have a high flow rate but relatively low O2 consumption, has an oxygen saturation of about 90%. By contrast, the blood in the venous drainage from the heart is only 25% saturated with O2. This is an important concept in relation to physiological control mechanisms and to the pathological consequences of disturbances of coronary blood flow (see Chapter 5). The shape and position of the oxyhaemoglobin dissociation curve (Fig. 1.4) shows one of the safety factors in relation to lung function. The top of the curve is nearly flat from 13 kPa (100 mm Hg), normal arterial Po2, down to about 10 kPa (75 mm Hg). This means that a decrease in Po2 within this range makes little difference to the % saturation of haemoglobin with oxygen, that is little change to the total amount of oxygen carried in arterial blood. Put another way, we can afford to have a certain degree of lung malfunction before it makes any significant difference to oxygen delivery to the tissues.
A DESIGN SPECIFICATION FOR THE CARDIOVASCULAR SYSTEM
Oxygen consumption
Diffusion
Carriage of oxygen in blood
Thoracic Key
Fastest Thoracic Insight Engine