Acute respiratory distress syndrome
A form of noncardiogenic pulmonary edema that causes acute respiratory failure, acute respiratory distress syndrome (ARDS), also called
shock lung or
adult respiratory distress syndrome, results from increased permeability of the alveolocapillary membrane. Fluid accumulates in the lung interstitium, alveolar spaces, and small airways, causing the lung to stiffen. Effective ventilation is thus impaired, prohibiting adequate oxygenation of pulmonary capillary blood. Severe ARDS can cause intractable and fatal hypoxemia. Patients who recover, however, may have little or no permanent lung damage. (See
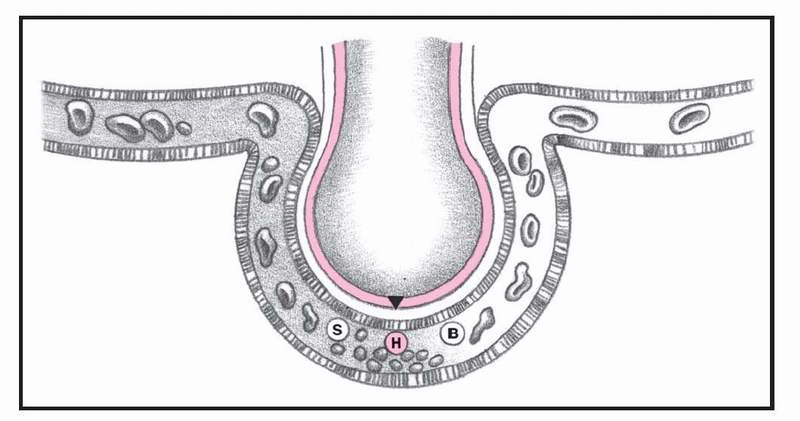
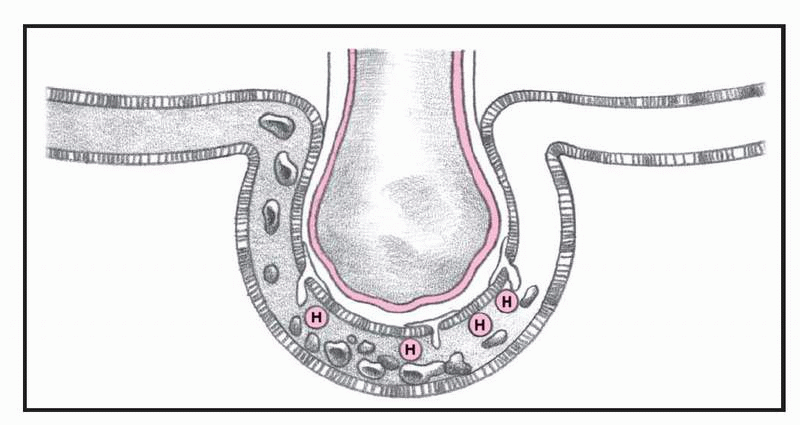
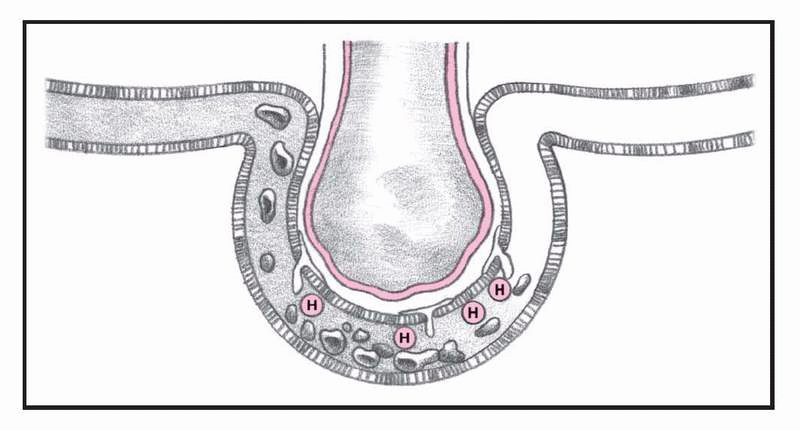
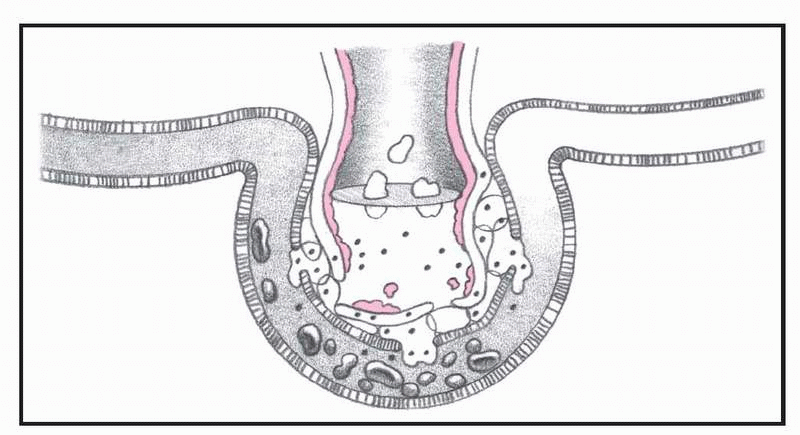
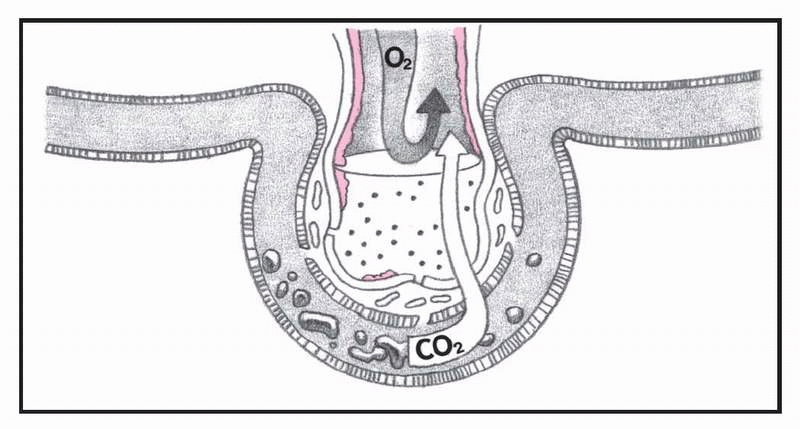
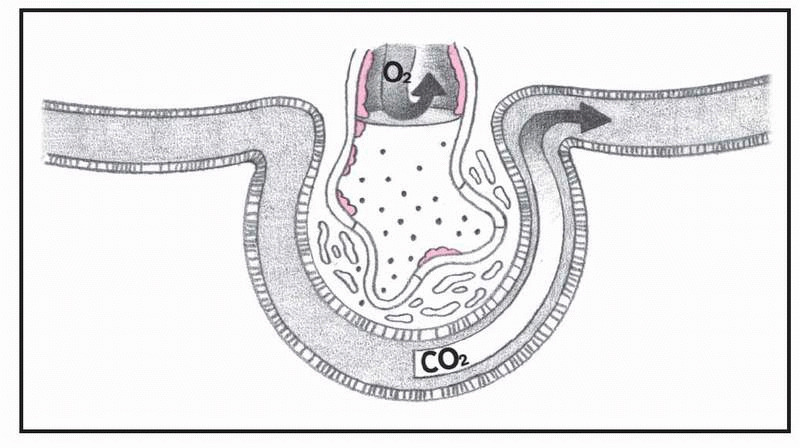
What happens in ARDS, pages 4-5.)
CAUSES AND INCIDENCE
ARDS affects 10 to 14 people per 100,000, with a mortality rate of 36% to 52%. The mortality rate remains high in the geriatric population in those aged 60 to 69 as shown by studies of trauma patients. Trauma is the most common cause of ARDS, possibly because trauma-related factors, such as fat emboli, sepsis, shock, pulmonary contusions, and multiple transfusions, increase the likelihood of microemboli developing.
Other common causes of ARDS include anaphylaxis, aspiration of gastric contents, diffuse pneumonia (especially viral), drug overdose (for example, heroin, aspirin, and ethchlorvynol), idiosyncratic drug reaction (to ampicillin and hydrochlorothiazide), inhalation of smoke or noxious gases (such as nitrous oxide, ammonia, and chlorine), near-drowning, and oxygen toxicity. Less common causes of ARDS include coronary artery bypass grafting, hemodialysis, leukemia, acute miliary tuberculosis, pancreatitis, thrombotic thrombocytopenic purpura, uremia, and venous air embolism.
In ARDS, altered permeability of the alveolocapillary membrane causes fluid to accumulate in the interstitial space. If the pulmonary lymphatic glands can’t remove this fluid, interstitial edema develops. The fluid collects in the peribronchial and peribronchiolar spaces, producing bronchiolar narrowing. Hypoxemia occurs as a result of fluid accumulation in alveoli and subsequent alveolar collapse, causing the shunting of blood through nonventilated lung regions. In addition, alveolar collapse causes a dramatic increase in lung compliance, which makes it more difficult to achieve adequate ventilation.
SIGNS AND SYMPTOMS
• Initially, rapid, shallow breathing and dyspnea within hours to days of the initial injury (sometimes after the patient’s condition appears to have stabilized)
• Hypoxemia, which causes an increased drive for ventilation
• Intercostal and suprasternal retractions resulting from the effort required to expand the stiff lung
• Fluid accumulation, which produces crackles and rhonchi
• Restlessness, apprehension, mental sluggishness, and motor dysfunction
• Tachycardia, possibly with transient increased arterial blood pressure
• Hypotension
• Decreasing urine output
• Respiratory and metabolic acidosis
• Eventually, ventricular fibrillation or standstill
COMPLICATIONS
• Multisystem failure
• Pulmonary fibrosis
• Pneumothorax
DIAGNOSIS
• On room air, arterial blood gas (ABG) analysis initially shows decreased partial pressure of arterial oxygen (PaO2) less than 60 mm Hg and partial pressure of arterial carbon dioxide (PaCO2) less than 35 mm Hg. The resulting pH usually reflects respiratory alkalosis.
• ABG analysis shows respiratory acidosis (increasing PaCO2 [more than 45 mm Hg]), metabolic acidosis (decreasing bicarbonate [less than 22 mEq/L]), and a decreasing PaO2 despite oxygen therapy as ARDS becomes more severe.
• Pulmonary artery blood shows decreased oxygen saturation, re-flecting tissue hypoxia.
• Serial chest X-rays initially show diffuse bilateral infiltrates that tend to be more peripheral and patchy, rather than the usual perihilar “bat wing” appearance of cardiogenic pulmonary edema.
• Later chest X-rays reveal ground-glass appearance and “whiteouts” of both lung fields as hypoxemia becomes irreversible.
Differential diagnosis must rule out cardiogenic pulmonary edema, pulmonary vasculitis, and diffuse pulmonary hemorrhage. To establish the etiology, laboratory work should include sputum Gram stain, culture and sensitivity tests, and blood cultures to detect infections; a toxicology screen for drug ingestion; and, when pancreatitis is a consideration, a serum amylase determination.
TREATMENT
When possible, treatment is designed to correct the underlying cause of ARDS as well as to prevent progression and the potentially fatal complications of hypoxemia and respiratory acidosis. Supportive medical care consists of administering humidified oxygen with continuous positive airway pressure. Hypoxemia that doesn’t respond adequately to these measures requires ventilatory support with intubation, volume ventilation, and positive end-expiratory pressure (PEEP). Other supportive measures include fluid restriction, diuretics, and correction of electrolyte and acid-base abnormalities.
Drugs
• Sedatives, opioids, or neuromuscular blocking agents to optimize ventilation when ARDS requires mechanical ventilation
• Sodium bicarbonate to reverse severe metabolic acidosis, although in severe cases this may worsen the acidosis if carbon dioxide can’t be cleared adequately
• Vasopressors and I.V. fluids to maintain blood pressure
• Antibiotics to treat infections
• Methylprednisolone infusions in early severe ARDS to significantly improve pulmonary and extrapulmonary organ dysfunction and reduce duration of mechanical ventilation and length of stay in the intensive care unit
• Anticoagulants and fibrinolytics (under study) to improve lung function and oxygenation in ARDS
SPECIAL CONSIDERATIONS
ARDS requires careful monitoring and supportive care.
• Frequently assess the patient’s respiratory status. Be alert for retractions on inspiration. Note the rate, rhythm, and depth of respirations; watch for dyspnea and the use of accessory muscles of respiration. On auscultation, listen for adventitious or diminished breath sounds. Check for clear, frothy sputum, which may indicate pulmonary edema.
• Observe and document the hypoxemic patient’s neurologic status (level of consciousness and mental status).
• Maintain a patent airway by suctioning, using sterile, nontraumatic technique. Ensure adequate humidification to help liquefy tenacious secretions.
• Closely monitor heart rate and blood pressure. Watch for arrhythmias that may result from hypoxemia, acid-base disturbances, or electrolyte imbalance. With pulmonary artery
catheterization, know the desired pressure levels. Check readings often and watch for decreasing mixed venous oxygen saturation.
• Monitor serum electrolytes and correct imbalances. Measure intake and output; weigh the patient daily.
• I.V. fluid management involves careful balancing. Fluids are necessary for oxygen and nutrient delivery; however, excessive fluid administration can worsen lung edema, further damaging gas exchange. Conservative fluid management has been shown to improve lung function as well as shorten duration of mechanical ventilation and I.V. care without increasing incidence of nonpulmonary organ failure.
• Check ventilator settings frequently, and empty condensate from tubing promptly to ensure maximum oxygen delivery. Monitor ABG studies and pulse oximetry. The patient with severe hypoxemia may need controlled mechanical ventilation with positive pressure. Give sedatives, as needed, to reduce restlessness.
• Because PEEP may decrease cardiac output, check for hypotension, tachycardia, and decreased urine output. Suction only as needed to maintain PEEP or use an in-line suctioning apparatus. Reposition the patient often and record an increase in secretions, temperature, or hypotension that may indicate a deteriorating condition. Monitor peak pressures during ventilation. Because of stiff, noncompliant lungs, the patient is at high risk for barotrauma (pneumothorax), which is evidenced by increased peak pressures, decreased breath sounds on one side, and restlessness.
• Prone positioning has been shown to reduce right ventricular overload. It permits an additional limitation in airway pressure (associated with a reduction in hypercapnia), reduces airway pressure, improves alveolar ventilation, and decreases right ventricular enlargement.
• Monitor nutrition, maintain joint mobility, and prevent skin breakdown.
• Accurately record calorie intake. Give tube feedings and parenteral nutrition, as ordered.
• Perform passive range-of-motion exercises or help the patient perform active exercises, if possible.
• Provide meticulous skin care.
• Plan patient care to allow periods of uninterrupted sleep.
• Provide emotional support. Warn the patient who’s recovering from ARDS that recovery will take some time and that he will feel weak for a while.
• Watch for and immediately report all respiratory changes in the patient with injuries that may adversely affect the lungs (especially during the 2- to 3-day period after the injury when the patient may appear to be improving).
Acute respiratory failure in COPD
In patients with essentially normal lung tissue, acute respiratory failure
(ARF) usually means partial pressure of arterial carbon dioxide (PaCO
2) above 50 mm Hg and partial pressure of arterial oxygen (PaO
2) below 50 mm Hg. These limits, however, don’t apply to patients with chronic obstructive pulmonary disease (COPD), who usually have a consistently high PaCO
2 and low PaO
2. In patients with COPD, only acute deterioration in arterial blood gas (ABG) values, with corresponding clinical deterioration, indicates ARF.
CAUSES AND INCIDENCE
The incidence of ARF increases markedly with age and is especially high among people age 65 and older.
ARF may develop in patients with COPD as a result of any condition that increases the work of breathing and decreases the respiratory drive. These conditions may result from respiratory tract infection (such as bronchitis or pneumonia), bronchospasm, or accumulated secretions secondary to cough suppression. Other common causes are related to ventilatory failure, in which the brain fails to direct respiration, and gas exchange failure, in which respiratory structures fail to function properly.
Central nervous system depression can occur due to head trauma or injudicious use of sedatives, opioids, tranquilizers, or oxygen. Cardiovascular disorders (myocardial infarction, heart failure, or pulmonary emboli) can result in untreated ventilation-perfusion ([V with dot above][Q with dot above]) imbalances, which lead to right-to-left shunting, in which blood passes from the heart’s right side to the left without being oxygenated. This unoxygenated blood reaches the arterial system and is distributed to the rest of the body. Likewise, hypoxemia deprives the myocardial tissue of oxygen and nutrients, possibly resulting in ischemia or a myocardial infarction. Thoracic abnormalities, such as chest trauma, pneumothorax, or thoracic or abdominal surgery can result in hypoventilation and resultant ARF in patients with COPD. Airway irritants, such as smoke and fumes, may produce hypoxia. Endocrine or metabolic disorders, such as myxedema or metabolic acidosis, place the patient with COPD at greater risk for ARF due to the patient’s limited ability to compensate with the respiratory system. Also, noncompliance with prescribed bronchodilator or corticosteroid therapy can result in ARF in the COPD patient.
SIGNS AND SYMPTOMS
• Decreased PaO2 (hypoxemia) and increased PaCO2 (hypercapnia) due to increased [V with dot above][Q with dot above] mismatch and reduced alveolar ventilation (as shown by ABGs); this rise in carbon dioxide lowers the pH.
• Increased, decreased, or normal respiratory rate, depending on the cause; shallow or deep respirations, or both; possible air hunger
• Possible cyanosis, depending on the hemoglobin (Hb) level and arterial oxygenation
• Crackles, rhonchi, wheezing, or diminished breath sounds as heard on auscultation
• Restlessness, confusion, loss of concentration, irritability, tremulousness, diminished tendon reflexes, papilledema, and eventual unresponsiveness (coma) as hypoxemia and hypercapnia occur
• Tachycardia, with increased cardiac output and mildly elevated blood pressure secondary to adrenal release of catecholamine, occurring early in response to low PaO2
• Arrhythmias with myocardial hypoxia
• Elevated pressures on the right side of the heart, distended jugular veins, an enlarged liver, and peripheral edema due to pulmonary hypertension, which occurs secondarily to pulmonary capillary vasoconstriction
• Cardiac fa• ilure due to stress on the heart
COMPLICATIONS
• Tissue hypoxia
• Metabolic acidosis
• Multiple organ failure
• Cardiac arrest
DIAGNOSIS
• Progressive deterioration in ABG levels and pH, when compared with the patient’s “normal” values, strongly suggests ARF in COPD. (In patients with essentially normal lung tissue, pH below 7.35 usually indicates ARF, but patients with COPD display an even greater deviation from this normal value, as they do with PaCO2 and PaO2.)
• Increased bicarbonate levels indicate metabolic alkalosis or reflect metabolic compensation for chronic respiratory acidosis.
• Abnormally low hematocrit (HCT) and Hb levels may be due to blood loss, indicating decreased oxygen-carrying capacity. Elevated levels may occur with chronic hypoxemia.
• Hypokalemia and hypochloremia may result from diuretic and corticosteroid therapies used to treat ARF.
• White blood cell count is elevated if ARF is due to bacterial infection; Gram stain and sputum culture can identify pathogens.
• Chest X-ray findings identify pulmonary pathologic conditions, such as emphysema, atelectasis, lesions, pneumothorax, infiltrates, or effusions.
• Arrhythmias on electrocardiogram commonly suggest cor pulmonale and myocardial hypoxia.
TREATMENT
ARF in patients with COPD is an emergency that requires cautious oxygen therapy (using nasal prongs or Venturi mask) to raise the PaO
2. In patients with chronic hypercapnia, oxygen therapy can cause hypoventilation by increasing PaCO
2 and decreasing the respiratory drive, necessitating mechanical ventilation. The minimum fraction of inspired oxygen (FIO
2) required to maintain ventilation or oxygen saturation greater than 85% to 90% should be used. If significant uncompensated respiratory
acidosis or unrefractory hypoxemia exists, mechanical ventilation (through an endotracheal [ET] or a tracheostomy tube) or noninvasive ventilation (with a face or nose mask) may be necessary. Postural drainage and chest physiotherapy is instituted to help clear secretions. For the nonventilated patient retaining carbon dioxide, encourage him to cough and breathe deeply, teach him pursed-lip and diaphragmatic breathing to control dyspnea, and have him use an incentive spirometer.
Drugs
• Bronchodilators to relax bronchial smooth muscle and improve air flow
• Corticosteroids to decrease airway inflammation (often used for 10 to 14 days only)
• Anti-infectives specific to treat infection
• Antacids, histamine-2 receptor antagonists, or sucralfate in the intubated patient to prevent or treat stress
SPECIAL CONSIDERATIONS
• Because most patients with ARF are treated in an intensive care unit, orient them to the environment, procedures, and routines to minimize their anxiety.
• To reverse hypoxemia, administer oxygen at appropriate concentrations to maintain PaO2 at a minimum of 50 to 60 mm Hg. Patients with COPD usually require only small amounts of supplemental oxygen. Watch for a positive response—such as improvement in the patient’s breathing, color, and ABG levels.
• Maintain a patent airway. If the patient is retaining carbon dioxide, encourage him to cough and to breathe deeply. Teach him to use pursed-lip and diaphragmatic breathing to control dyspnea. If the patient is alert, have him use an incentive spirometer; if he’s intubated and lethargic, turn him every 1 to 2 hours. Use postural drainage and chest physiotherapy to help clear secretions.
• In an intubated patient, suction the trachea, as needed, after hyperoxygenation. Observe for a change in quantity, consistency, and color of sputum. Provide humidification to liquefy secretions.
• Observe the patient closely for respiratory arrest. Auscultate for breath sounds. Monitor ABG levels and report any changes immediately.
• Check the cardiac monitor for arrhythmias.
• If the patient requires mechanical ventilation:
— Check ventilator settings, cuff pressures, and ABG values often because the FIO2 setting depends on ABG levels. Draw specimens for ABG analysis 20 to 30 minutes after every FIO2 change or oximetry check.
— Prevent infection by using sterile technique while suctioning.
— Stress ulcers are common in the intubated patient. Check gastric secretions for evidence of bleeding if the patient has a nasogastric tube or
if he complains of epigastric tenderness, nausea, or vomiting. Monitor Hb level and HCT; check all stools for occult blood. Administer antacids, histamine-2-receptor antagonists, or sucralfate, as ordered.