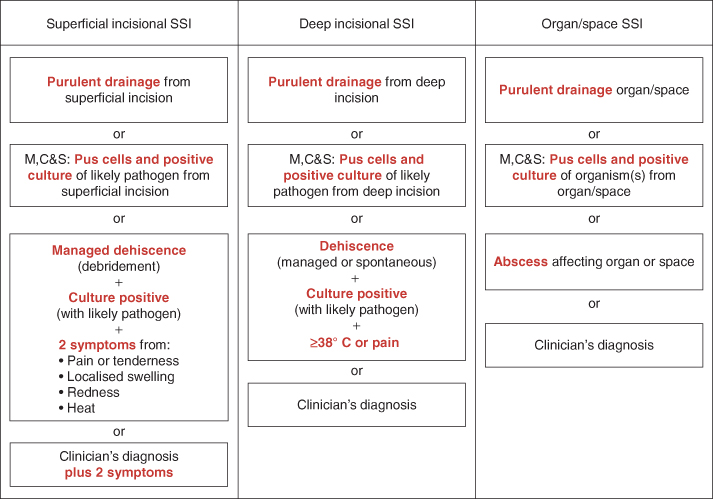
Figure 7.1 Surgical site infection: definition and classification adapted from Health Protection Agency protocol. Adapted from HPA protocol (2011).
Vascular response
Following the creation of a wound, the body seeks to rebalance itself (return to homeostasis). The aim of the microvascular response is to stop the bleeding (haemostasis) and to protect the area against foreign material or the ingress of harmful bacteria.
This is achieved via attributes of the blood vessel walls (the endothelium) as well as properties of the blood. Blood vessels constrict or narrow, which reduces blood flow to the area. Platelets (small cell fragments) gather together at the site. Chemical signals direct vascular and cellular activities to create a mesh of plasma proteins – predominantly fibrin and fibronectin – which trap red blood cells, resulting in coagulation (clotting) to stop the bleeding (Bale 2004). In addition, the clot provides a rudimentary barrier to bacteria and forms a basic structure upon which the cellular response will build (Benbow 2005).
After haemostasis, further microvascular events serve to dilute any toxins in the area and to facilitate the cellular response:
- The blood vessels dilate – arteriolar smooth muscle relaxes so that the blood flow to the small blood vessels (the capillaries and venules) is increased.
- Mild hyperaemia (an increase in haemoglobin or red blood cells) results – this brings oxygen, warmth and protective cells to the injured area (Woolf 2000, Collier 2004). The hyperaemia accounts for the heat and redness observed at the wound site.
- The permeability of small vessels increases – intercellular pores expand in order to create temporary gaps in the endothelium (Walsh 2004). Together, the increased blood flow (which causes pressure on the small vessels and fluid to escape into the tissue) and the increased permeability of the blood vessels account for the oedema (swelling) and pain at the wound site.
- Pain is stimulated either by the direct trauma or swelling due to the increase in tissue tension, and/ or the release of chemical stimulants (Walsh 2004).
- When of sufficient magnitude, both swelling and pain may result in what may be considered the fifth sign of inflammation – loss of function.
Cellular response
The tissue injury and the clotting factors stimulate the inflammatory cells, cytokines and other proinflammatory substances (such as prostaglandin, bradykinin and histamine) (Benbow and Stevens 2010). In order to coordinate their efforts, inflammatory cells need to send signals to each other and they do this by releasing cytokines (molecules which attract and stimulate cells).
Facilitated by the vascular response, a restorative and protective exudate of fluid, colloid particles and larger molecules of the immune defences (antibodies and complement) can then move out of the vessel and into the local tissue (Benbow and Stevens 2010). The clearance of exudate, dead tissue, debris and bacteria is reliant on phagocytosis, which is the engulfment and destruction of the microbe by inflammatory cells (Male 2007). Phagocytosis is performed by white blood cells (WBCs).
WBCs may reside in the tissue (polymorphonuclear leukocytes or ‘polymorphs’) or they may circulate in the system and are mobilised to areas of acute inflammation (neutrophils and monocytes – monocytes later change into macrophages). The purposeful movement of phagocytic cells towards the site is called chemotaxis. The presence of bacteria attracts more neutrophils to the area than would the creation of the wound (Woolf 2000).
In a wound healing by primary intention, this phase is usually completed in 3 days. However, heavy bacterial contamination, impaired immunity, underlying illness, poor vascularity or perfusion may all prolong the inflammatory process.
Proliferative or reconstructive phase
Lakhani and Dogan report that ‘the process of repair requires many different cell types to proliferate and synthesize proteins necessary for restoring integrity and strength to the tissue’ (Lakhani and Dogan 2004, p. 322). Two to four days after surgical incision, as the clearance of bacteria and debris finishes, macrophages attract fibroblasts (connective tissue cells) and myofibroblasts to the area to deposit collagen (Gould and Brooker 2000). The different types of collagen (the main supportive protein in the body) and extracellular substances provide the framework (extracellular matrix) for new tissue and angiogenesis (the creation of new blood vessels) to take place.
Granulation tissue, made up of inflammatory cells and fibroblasts, fills the wound from the base upwards and is usually not evident in wounds healing by primary intention; this process is more laborious and of a larger scale in wounds healing by second intention (Woolf 2000, Vuolo 2010).
The vascularity of the area produces bright red, granular tissue which bleeds easily because of the newly formed capillaries present (Lakhani and Dogan 2004).
The wound margin or border will be pulled together, which reduces the overall size of the wound; however contraction is minimal in wounds healing by primary closure (Bryant and Nix 2007). Concurrently, cells migrate and proliferate to cover over the wound. Within 24 hours epithelial cells are present on clean surgical wounds (Mercandetti and Cohen 2005), although healing will still be taking place below (Sheperd 2009). It should be noted epithelial cells will not form over eschar or necrotic tissue.
The new epithelial cells are white/pink in appearance and are vulnerable to shear forces.
Maturation phase
During this phase, the wound becomes less vascular. Over time, the structure and strength of the new tissue is improved (Gould and Brooker 2000).
Types of Wound Closure
The type of suture/closure material, the use and type of needle delivery system and suturing technique (method and stitch size) all depend on where the suture is placed, the features of the closure material and the condition of the patient. Sutures may be placed in a variety of manners, for instance using continuous or interrupted (e.g. knotted) methods such as the ‘interrupted mattress’, ‘percutaneous’ or ‘transdermal’ techniques, or using the more complex figure of eight or subcuticular techniques (NICE 2008).
Wound healing by primary intention
Wounds healing by primary intention have their incisions closed directly following the operation, with sutures, staples or adhesives (Table 7.1). This type of closure is applied in instances where there is little tissue loss and the wound edges can be brought together. Such wounds generally result in minimal oedema, gaping and serous discharge (Gottrup et al. 2005). Table 7.2 provides further comment. This method is suitable for clean, clean-contaminated or traumatic wounds if thorough cleansing/debridement of the wound is achieved (Gould and Brooker 2000).
Table 7.1 Wound closure materials.
| Absorbable | Non-absorbable |
| Natural | Natural |
| Synthetic | Surgical silk |
| Coated vicryl | Synthetic |
| Monocryl | Nylon |
| Polydioxanone (PDS) II | Polypropylene (Prolene) Tissue adhesive (e.g. Dermabond®, Bioglue®) Tapes/strips (e.g. Steri-strip®) |
Table 7.2 Surgical wounds.
| Recommendation | Comment | |
| Incisions | The incision is protected by dressing and /or sealant | The skin should be allowed to seal and resume its natural protective role against bacterial ingress – only a minority of pathogens can penetrate intact skin (Roitt et al. 1993). Edward-Jones (2010) notes microbes rarely penetrate intact skin, and if they do the resultant infection is likely to be systemic rather than local wound infection |
| Compression may be required to reduce bleeding, exudate, oedema or haematoma formation | Fold a sterile surgical pad or layers of gauze into a narrow strip (which provides extra height area – to improve the mechanism for pressure) over the original wound dressing and either encircle with a crepe bandage (50% overlap) or apply an adhesive dressing stretched over and onto the skin (David et al. 2010). If bleeding is not stemmed, continue to apply firm pressure on dressing/additional padding and seek surgical advice After approximately 72 hours, the presence of serous exudate is no longer contributing to the healing process (Wilson 2003). Wound management choices should avoid both frequent dressing changes and maceration of the surrounding skin/ tissue breakdown. Seek advice from tissue viability nurse or surgeon (Sheppard and Wright 2006) Wound margins under strain may benefit from additional suture or adhesive tape (for instance Steristrips) (NICE 2008) but equally re-suturing, support resources (e.g. cough lock, bras for females with sternal wounds), appropriate fluid balance management and counter-gravity measures (e.g. elevating the affected limb) may be appropriate | |
| Dressings | Dressings which allow the wound to be viewed, and offer good conformability, patient comfort and which do not requiring frequent changing (allowing the patient to shower with the dressing remaining intact) are ideal (NICE 2008) | The dressing application must allow for postoperative oedema (either of the wound and/or general) and patient movement otherwise the skin may blister (Pukki et al. 2010 Dressings with a favourable vapour transmission rate and which promote moist wound healing (see Box 7.2) should be selected (NICE 2008) |
| Drains | Surgical incisions may have drains (e.g. Redivac, Bellovac) placed distally through a separate incision | This is to reduce the risk of complications including dehiscence due to pressure on the suture line and to improve healing by countering dead space and haematoma formation (Gruendemann and Mangum 2001). Drain entry sites need to be managed aseptically to reduce introduction of bacteria. Output from the drains should be monitored and reported and the drains should be removed as soon as their purpose has been served |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


