CASE 7 Saphenous Vein Graft Disease
Case presentation
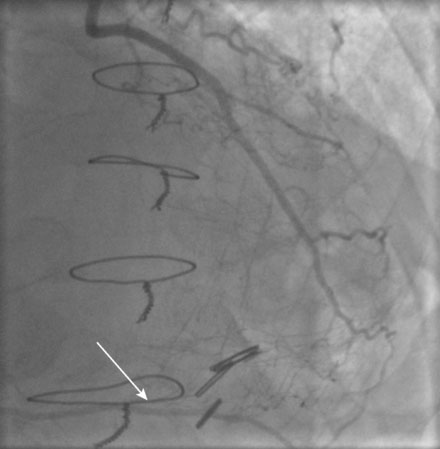
He had known left ventricular dysfunction with an ejection fraction of 40%, but had remained asymptomatic since his bypass operation until 2 to 3 weeks before presentation, when he noted progressive shortness of breath with any exertion associated with chest tightness. He developed rest dyspnea and then became unable to lie flat without developing a sense of suffocating. He presented to the emergency room and was promptly admitted with a diagnosis of congestive heart failure and unstable angina. His electrocardiogram showed nonspecific abnormalities that were unchanged from prior ECGs, and serial troponin assays remained in the normal range. However, an echocardiogram showed deterioration in his left ventricular function with an ejection fraction of 15% to 20%. He subsequently underwent cardiac catheterization, which found a chronically-occluded right coronary artery and a patent left internal mammary to the LAD with large collateral vessels to the right coronary (Figure 7-1 and Video 7-1). The native proximal LAD and circumflex arteries were completely occluded. The saphenous vein graft to the ramus had a very severe stenosis located in the proximal segment near the aortic anastomosis (Figures 7-2, 7-3 and Videos 7-2, 7-3). He was referred for percutaneous coronary intervention of the saphenous vein graft.
Cardiac catheterization
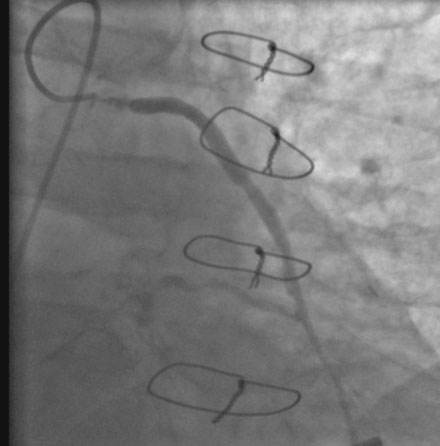
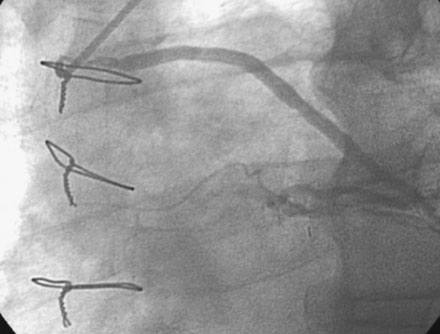
The night prior to the procedure, the patient received a loading dose of 600 mg clopidogrel and, after obtaining arterial access, the operator administered bivalirudin as the procedural anticoagulant. A 6 French Judkins right 4.0 guide catheter was engaged in the saphenous vein graft. To achieve distal embolic protection, the operator advanced a filter wire past the stenosis and positioned the filter in the distal portion of the vein graft (Figure 7-4). The lesion in the proximal vein graft was first treated with a 3.0 mm diameter by 20 mm long compliant balloon and then with a 4.0 mm diameter by 23 mm long bare-metal stent. The stent was postdilated with a 4.5 mm diameter noncompliant balloon to high atmospheres. The filter wire was retrieved and angiography showed normal flow in the ramus with no evidence of distal embolization and an excellent luminal result. The final angiographic results are shown in Figure 7-5 and Video 7-4.
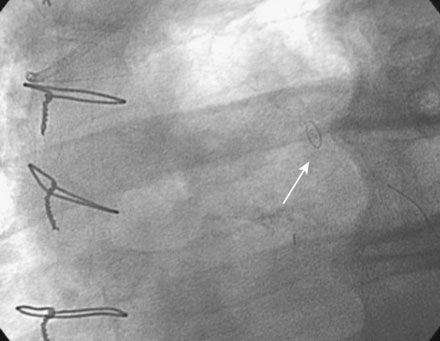
FIGURE 7-4 Distal embolic protection was accomplished with a filter wire positioned in the vein graft (arrow).
Postprocedural course
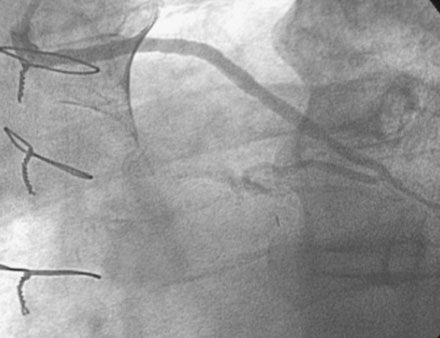
He had no postprocedure complications and was discharged the next morning on lisinopril, atenolol, furosemide, rosuvastatin, aspirin, clopidogrel, and insulin. At follow-up visits 6 weeks and 3 months later, he remained free of chest pain, orthopnea, or significant dyspnea on exertion. Repeat echocardiography found no change in his severe left ventricular dysfunction, and his physician planned to refer him for an implantable defibrillator. However, 4 months after the intervention, he experienced recurrent chest tightness and presented to the emergency room after a prolonged episode. He became pain-free after nitroglycerin administration, and his troponin peaked at 4.0 ng/mL. Repeat catheterization showed no change in the left internal mammary to the LAD, but severe focal in-stent restenosis was seen in the saphenous vein graft to the ramus (Figure 7-6 and Video 7-5). Again, a distal embolic protection device was placed and intervention performed first with a 3.5 mm balloon (Figure 7-7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree