History
A 53-year-old man of Greek origin was diagnosed with dilated cardiomyopathy in 1996, at which time his left ventricular ejection fraction (LVEF) was described as moderatelely to severely reduced and coronary artery disease was initially excluded by angiography. The patient was in New York Heart Association (NYHA) class II and received beta blockers and angiotensin-converting enzyme inhibitors. Cardiac risk factors included arterial hypertension, medically treated type 2 diabetes mellitus, and ongoing smoking. His family history showed no cases of cardiomypathy or sudden cardiac death. His medical history included thalassemia minor (with normal hemoglobin values), hypothyroidism, and chronic gastritis.
In September 2001 the patient was diagnosed with stage III Hodgkin’s lymphoma with involvement of mediastinal and cervical lymph nodes and the spleen. In the ensuing months he received chemotherapy according to a modified escalated protocol of bleomycin, etoposide, doxorubicin (Adriamycin), cyclophosphamide, oncovin-vincristine, procarbazine, and prednisone (BEACOPP), followed by radiation therapy. Cardiotoxic anthracyclines were avoided because of the underlying heart condition. Insulin therapy was started for his worsening diabetes.
Over the next few years the patient was followed closely at regular intervals in the oncology outpatient clinic, with imaging studies that excluded relapse of the lymphoma. Echocardiographic and clinical evaluation findings of the heart were stable during this time.
In February 2009 the patient was admitted to the hospital with cardiac decompensation and received intravenous diuretic therapy. Magnetic resonance imaging showed an LVEF of 20% and a left ventricular end-diastolic dimension of 81 mm. An electrocardiogram (ECG) showed normal sinus rhythm with a left bundle branch block (LBBB) and a QRS width of 128 ms. The patient was offered implantation of a biventricular implantable cardioverter-defibrillator (CRT-D), which he refused.
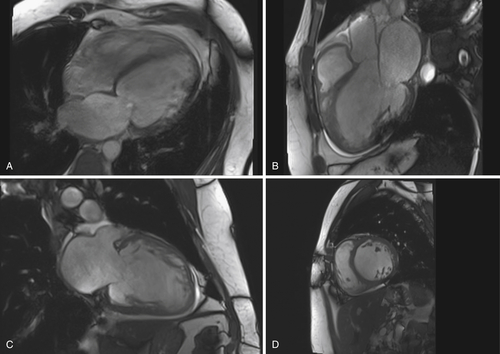
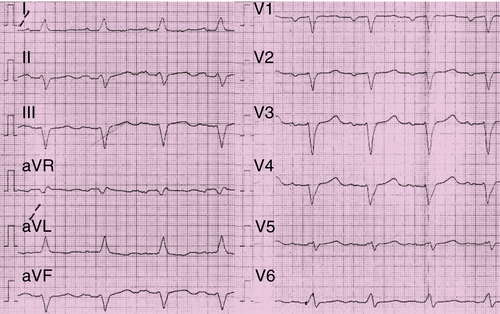
Over the next 2 years the patient’s physical condition deteriorated further despite extensive treatment with heart failure medications. In February 2011 he was hospitalized because of progressive dyspnea at rest. Chest progressive radiography revealed pleural effusions. Cardiac catheterization demonstrated two-vessel coronary artery disease with a peripheral occlusion of the left anterior descending artery. A percutaneous coronary intervention was not possible. Magnetic resonance imaging demonstrated a markedly reduced LVEF of 11% (Figure 41-1). ECG findings were unchanged in contrast to those in 2009 with sinus rhythm, borderline LBBB, a QRS width of 130 ms, and a QRS onset to peak R duration of 60 ms in V5 and V6 (Figure 41-2). The patient finally consented to the implantation of a CRT-D device, which was performed in February 2011. An atrial electrode (Flextend 2, Boston Scientific, Natick, Mass.) was placed in the right atrial appendage, the right ventricular lead (Endotak Reliance SG, Boston Scientific) was placed in the right ventricular apex, and the left ventricular lead (Acuity Steerable, Boston Scientific) in a posterolateral vein. Good lead impedances and pacing and sensing thresholds were achieved for all three leads. The device parameters were programmed as follows: DDD pacing mode, tracking rate 60 to 130 bpm, paced atrioventricular interval of 130 ms, simultaneous biventricular stimulation with VV delay of 0 ms, and left ventricular electrode configuration of left ventricular tip to left ventricular ring. Despite stable stimulation percentage rates (95%-98%), little change occurred in the patient’s physical ability over the following months.
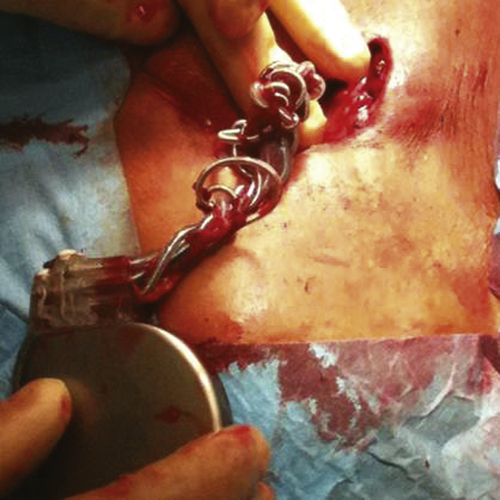
In August 2011 the patient’s fatigue and dyspnea were worsening. Interrogation of the implantable cardioverter-defibrillator (ICD) revealed abnormalities with the atrial lead. Impedance values and pacing and sensing thresholds with the right and left ventricular leads were stable. Chest radiography revealed Twiddler’s syndrome (Figure 41-3), with the atrial electrode drawn back into the left subclavian vein. The patient admitted that he had touched and rotated the device frequently. During the operative revision the device was observed to have been rotated around its axis 18 times. The device was repositioned, and the right atrial electrode was revised successfully.
In February 2012 the patient was hospitalized again because of cardiac decompensation and dyspnea at rest. He had ankle edema and pleural effusions. Device interrogation did not reveal significant ventricular or supraventricular tachyarrhythmias. The patient again required intravenous diuretic therapy.
Although cardiac compensation was achieved, the patient again developed dyspnea after walking only a few meters. His quality of life was measured with a 21-item scale according to the Minnesota Living with Heart Failure Questionnaire, with a score of 79. The N-terminal probrain natriuretic peptide value was elevated to 12.067 ng/L. An exercise test showed highly decreased VO2 peak value of 10.7 mL/kg/min with maximum exercise capacity of 40 watts.
The patient’s baseline medications, symptoms, physical examination results, laboratory data, and echocardiography are discussed in the following section. The patient ultimately underwent implantation of an Optimizer III device (Impulse Dynamics, Stuttgart, Germany) in March 2012, the results of which will be reviewed in detail.
Current Medications
The patient was taking atorvastatin 40 mg daily, carvedilol 37.5 mg daily, torsemide 40 mg daily, spironolactone 12.5 mg daily, enalapril 7.5 mg daily, opipramol 50 mg daily, pantoprazole 20 mg daily, mixed insulin 30 units daily, and aspirin 100 mg daily.
Current Symptoms
The patient was experiencing dyspnea on minimal effort and fatigue.
Physical Examination
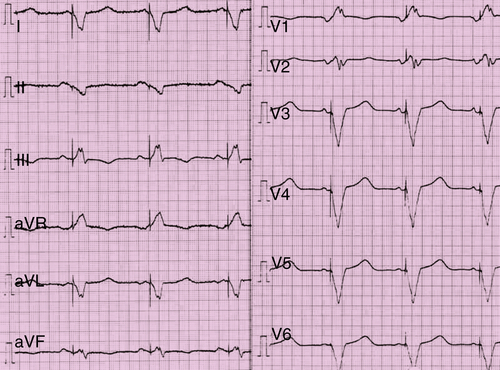
Laboratory Data
Electrocardiogram
Findings
The ECG showed normal rate and normal sinus rhythm, LBBB with a QRS width of 128 ms, and QRS onset to peak R time of 60 ms in V5 and V6 (see Figure 41-2).



