History
A 63-year-old man presented to the emergency room with recent-onset episodic chest discomfort described as “muscle ache.” The chest discomfort was radiating to the upper neck and both arms, associated with a tingling sensation. It was not reproducible or worsened on deep inspiration. He recently noticed dyspnea with minimal effort without any associated chest discomfort. However, he denied orthopnea, paroxysmal nocturnal dyspnea, pedal edema, and loss of consciousness.
A year previously, he was diagnosed with coronary artery disease, requiring a bare metal stent insertion for revascularization of 95% obstructive stenosis in the proximal left anterior descending coronary artery. His left ventricular ejection fraction (LVEF) was 38% at the time, and his electrocardiogram (ECG) revealed left bundle branch block (LBBB) morphology. His medical history included 9 months of chemotherapy for non-Hodgkin’s lymphoma. Approximately 4 years before the current visit, he was diagnosed with right cavernous meningioma, which was successfully treated with stereotactic radiation therapy without mantle radiation. During the same year, he was treated for benign prostatic hypertrophy with transurethral resection of prostate. He was being treated for depression, anxiety disorder, and hypothyroidism. The patient was married for 35 years and had two grown children. He reported stress at work, was a nonsmoker, and consumed alcohol socially. During the period of chemotherapy, he used marijuana. He had two sisters, who had cardiomyopathy of unclear cause. Both of his sisters died young, at 16 and 42 years of age. The patient’s niece was diagnosed with cardiomyopathy at the age of 38 and eventually needed cardiac transplantation.
Current Medications
The patient was taking levothyroxine 175 mcg daily, metoprolol 25 mg twice daily, bupropion 150 mg twice daily for depression, valsartan 160 mg daily, hydrochlorthiazide 12.5 mg daily, atorvastatin 40 mg daily, lorazepam 0.5 mg three times daily as needed for anxiety, zolpidem 5 mg daily at bedtime as needed for insomnia, and aspirin 325 mg daily.
Current Symptoms
The patient’s current symptoms were chest discomfort of 2 weeks, exertional dyspnea, and reduced exercise tolerance. On examination, he was overweight and not in any apparent distress and had a temperature of 36.8° C (98.2° F) and oxygen saturation of 99% on room air. The neurologic examination was nonfocal.
Physical Examination
Laboratory Data
Electrocardiogram
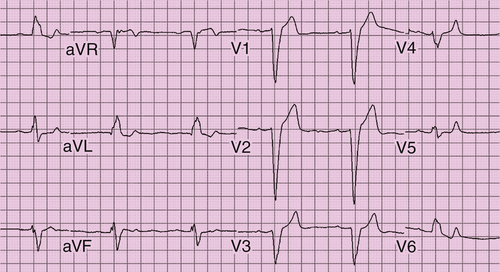
The ECG showed sinus bradycardia at a rate of 55 bpm, first-degree atrioventricular block (PR interval of 210 ms), LBBB unchanged in contrast to ECG obtained 1 month earlier. The QRS duration was 164 ms. No ST-T changes suggestive of ischemia were noted (Figure 38-1).
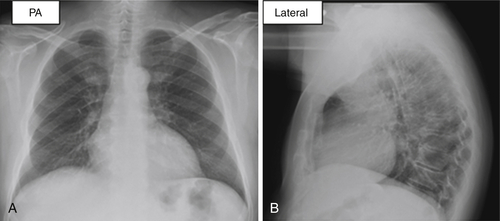
Chest Radiograph
The posteroanterior and lateral radiographic views demonstrated poor inspiration, mild cardiomegaly, and no evidence of infiltrate or effusion (Figure 38-2).
Comments
The patient’s chest discomfort and exertional dyspnea in the setting of prior coronary artery disease were concerning for acute coronary syndrome. It was reassuring that the initial myocardial markers were negative. Most important, the patient was not in acute heart failure.
Echocardiogram
The transthoracic echocardiogram showed normally functioning valves in mitral, aortic, tricuspid, and pulmonary positions. The left ventricle was diffusely hypokinetic with some regional variation, which was especially worse in the septum and apex. The LVEF diminished further to 32% in contrast to 38% 8 months previously.
Comments
The patient was admitted to the cardiac care unit. The serial cardiac enzyme examinations and ECGs showed no evidence of acute myocardial infarction.
Exercise Testing
The patient was able to complete a technetium-99m single-photon emission computed tomography (SPECT) myocardial perfusion scan. The exercise test was terminated because of the development of 2:1 atrioventricular block with hypotension. The SPECT perfusion imaging showed fixed perfusion defects in the anterior and septal segments. The left ventricle was dilated, demonstrating global systolic dysfunction.
Cardiac Catheterization
Subsequently, coronary angiography was performed that showed no evidence of obstructive coronary artery disease. The proximal left anterior descending artery with a prior bare metal stent was patent. The right coronary artery, the left main coronary artery, and the left circumflex artery showed minor luminal irregularities. No obstructive lesions were present.
Cardiac Magnetic Resonance Imaging
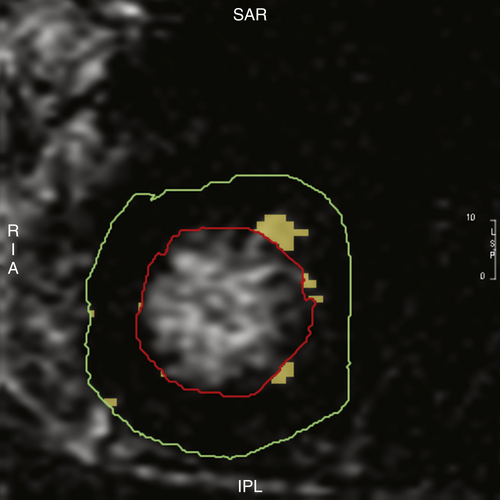
Cardiac magnetic resonance (CMR) with and without gadopentetate dimeglumine showed no evidence of myocardial edema. Delayed enhancement was seen in the subendocardial region of mid-anterior, anterolateral, and lateral apical segment consistent with scar. Scar extent was about 2% of left ventricular mass (Figure 38-3).
Final Diagnosis
The patient’s final diagnosis was mixed ischemia and nonischemic cardiomyopathy.
Focused Clinical Questions and Discussion Points
Question
What is the best management strategy to minimize the risk for sudden cardiac death in this patient?
Discussion
The patient had cardiomyopathy with an LVEF of 32% associated with LBBB in the setting of coronary artery disease, as well as New York Heart Association (NYHA) class II heart failure. Further, an added burden of conduction system disease manifested as first-degree atrioventricular block and LBBB on baseline ECG and development of Mobitz type 2 atrioventricular block with exercise, were suggestive of atrioventricular nodal or infranodal disease. In addition, the patient had a family history of cardiomyopathy and sudden cardiac death. Overall, he was at high risk for sudden cardiac death. He met the class I indication for pacing and the primary prevention criteria for implantable cardioverter-defibrillator (ICD) implantation.7 Increasing the medical therapy, especially beta blocker dosage, was not a viable option because of the bradycardia and relative hypotension. Pacemaker implantation will allow increasing the dosage of beta blockers but not address the sudden cardiac death risk. Overall, the patient has cardiomyopathy with low LVEF and dyssynchrony, as indicated by LBBB and wide QRS that can be best managed with cardiac resynchronization therapy with backup defibrillator (CRT-D).
Question
What further investigation will help in the decision-making process?
Discussion
The patient’s cardiomyopathy was much more advanced than the degree of coronary artery disease would explain, raising the possibility of the coexistence of idiopathic dilated cardiomyopathy or sarcoidosis or chemotherapy-induced cardiomyopathy. CMR will not only assess cardiac substrate with better resolution but also determine the scar burden and the scar location. Delayed-enhancement CMR (DE-CMR) or Late Gadolinium Enhancement (LGE)-CMR has been demonstrated to identify and quantify the scar accurately. CMR myocardial tagging can perform radial strain analysis to identify areas of dyssynchrony. An equilibrium contrast CMR can assess diffuse myocardial scar. It is also able to assess coronary venous anatomy that would help identify a suitable branch in an optimal left ventricular segment for left ventricular lead implantation. In addition, CMR is the gold standard for determining left ventricular function and volumes.
Mixed ischemic and nonischemic cardiomyopathy with atrioventricular nodal and intraventricular conduction system disease.
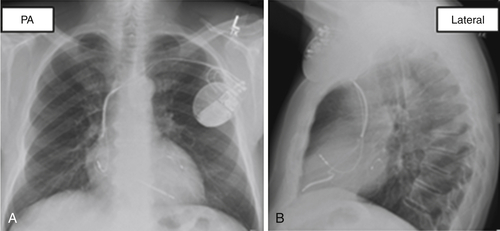
Question
What factors influenced the beneficial effect of CRT in this patient?
Discussion
The presence of LBBB, wide QRS duration (>150 ms), absence of atrial fibrillation, and optimal left ventricular lead location in a basal lateral segment are predictors of clinical response and left ventricular reverse remodeling.8




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


