Extensive work has also been undertaken by the Oxford Pain Research Group www.medicine.ox.ac.uk/bandolier/painres/PRintro.html) and the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine (ANZCA 2010) who regularly publish guidance on the evidence for acute pain management.
Physiology of Acute Pain
Pain may be considered as either nociceptive or neuropathic. Nociceptive is a term that describes the normal physiological processes relating to tissue damage, while neuropathic relates to pain caused by damage to or dysfunction of the nervous system (Briggs 2010). What a patient perceives as pain is a complex mix of physiology, previous experiences, culture and emotion.
Nociceptive pain
This type of pain has a protective function and alerts the person experiencing it to potential damage. It provides a reminder to postoperative patients to limit their movements and actions while their wound is healing. A painful stimulus (the surgical incision) is converted to an electrical signal in the nerve cells, which is known as transduction. Damaged cells release a number of chemical neurotransmitters including prostaglandins, serotonin and histamine, which sensitise these nerve endings. The electrical signal is then relayed to the dorsal horn in the spinal cord by two types of peripheral nerves (A-delta and C fibres). A-delta fibres are myelinated and quickly carry sharp, stabbing pain signals. Dull and throbbing pain is carried by the slower unmyelinated C fibres. This process is known as transmission.
These electrical signals are assisted by neurotransmitters so that the signal passes up the spinothalmic and spinoreticular tracts (ascending pathways) in the spinal cord to the brainstem and the thalamus in the brain (Briggs 2010). The information is then processed and relayed to other brain regions. The brainstem produces an autonomic response which can be seen in the patient and is illustrated in Table 24.1.
Repeated exposure to noxious stimuli such as cannulation can sensitise patients to further painful interventions. The body is able to modulate the pain experience by producing naturally occurring substances that can inhibit pain. These endogenous opioids include endorphins, enkephalins and dynorphins (Bromley 2005).
Table 24.1 Multi-dimensional effects of acute pain.
Adapted with permission of RCN Publishing from Briggs (2010).
| Physiological effects | Cognitive and emotional responses |
|
|
The descending pathways from the brain into the dorsal horn of the spinal cord can act as a gate (Melzack and Wall 2008). Opening the gate amplifies the pain signal while closing the gate can reduce the pain signal. Modulation of pain is not just reliant upon electrical signals and the action of analgesics as sensory input (e.g. distraction or relaxation) can reduce the intensity of the pain experience.
Neuropathic pain
This pain is often described by patients as burning, stinging, pricking, tingling or numbness. Neuropathic pain arises from dysfunction of the nerves. Patients may also report paroxysmal pain that may be shooting, stabbing or jabbing in nature. Neuropathic post-surgical pain may also be associated with allodynia (pain evoked by a normally non-painful stimulus such as clothing lightly touching the skin), hyperalgesia (an exaggerated pain response) and autonomic dysfunction (e.g. changes in skin temperature, blood flow and sweating). There are a number of validated tools to screen and diagnose neuropathic pain (Bouhassira and Attal 2011).
Pain Assessment
Hagger-Holt (2009) reminds us that pain is a subjective experience and is influenced by psychosocial factors and a patient’s previous experience of pain. Quantifying pain, using a unidimensional tool which looks at only one aspect of the patient’s pain experience, that is pain intensity using a numerical rating scale (where 0 = no pain, 10 = worst pain imaginable) is useful but alone does not provide sufficient information to determine the nature and properties of the pain nor its impact upon activities.
Pain after surgery at rest is usually moderate (i.e. 3–4 out of 10) for the initial two to three days, even when parenteral analgesia has been given. Pain at rest tends to resolve in the seven days after surgery, but pain on movement can be moderate or severe and persist for many weeks after surgery (Brennan 2011).
A multi-dimensional tool should be used at each patient assessment. A multi-dimensional tool is likely to assess the following factors:
- pain intensity (how strong is the pain?)
- location (where is the pain?)
- radiation
- triggers (what makes it worse?)
- alleviators (what makes it better?)
- pattern (brief, intermittent, constant)
- nature (e.g. dull, aching, sharp, stabbing).
An example of a multi-dimensional pain assessment tool is shown in Figure 24.2. Using the same tool at each assessment will determine whether the patient has experienced any improvement in their pain and functional ability. An ideal tool should has the following properties (Brown 2009). It should:
- be understood by patient and staff
- be quick to apply
- be consistently applied and evaluated with patient input
- give consideration to context and behavioural signs
- offer sensible, reliable and valid measures.
Figure 24.2 An example of a multi-dimensional pain assessment tool. Top part adapted from Melzack R (1975) The McGill Pain Questionnaire: major properties and scoring methods. Pain 1: 277–299. Lower part adapted from Bourbonnais F (1981) Pain assessment: development of a tool for the nurse and the patient. Journal of Advanced Nursing 6(4): 277–282.
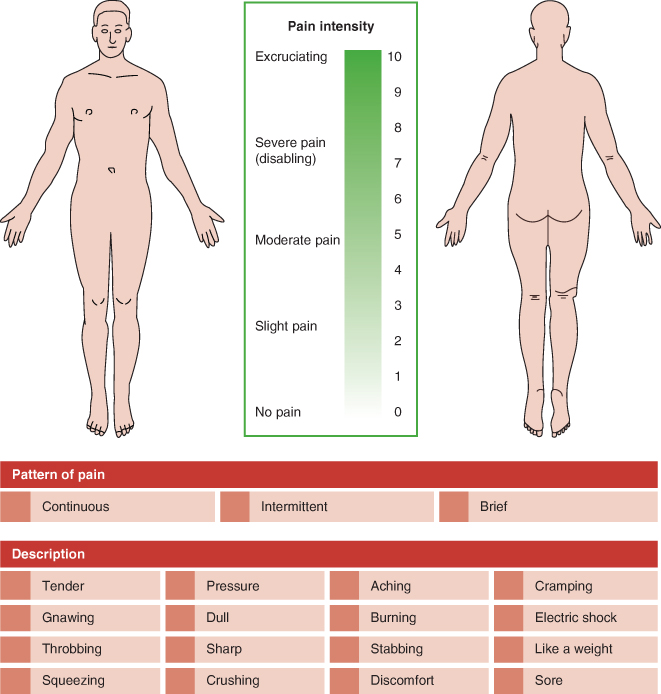
Pain assessment ideally should involve the patient but this can be challenging in non-verbal patients. Assessing pain in neonates requires the use of an observational tool that categorises behaviour (crying, grimacing) and physiology (heart rate, blood pressure). Chapters 25 and 26 describe the specific care of neonates and paediatrics.
Brown (2009) states that assessing pain in patients with moderate to severe cognitive impairment such as an older person with dementia necessitates observation of:
- physiological changes (vital signs, colour, sleep pattern, guarding, loss of appetite)
- body language changes (agitation, aggression, increased or decreased movement) and
- behavioural changes (facial expression, assuming the fetal position).
Patients may be unable to adequately express their pain verbally so the practitioner should observe behaviours to gain an understanding of the patient’s pain. The perioperative care of older people is described in Chapter 28.
The Biopsychosocial Model of Pain
Cognitive behaviour therapy (CBT) remains the most important contemporary psychological treatment for pain and is a class of treatments because it includes psychological and sociological aspects of the pain experience. It explores the meaning, behaviours and distress associated with pain by individual patients. Preoperative preparation and information giving can impact positively upon the patient’s experience and outcome. Preoperative preparation can consist of information about sensations (what can be expected, the nature and duration of pain), what behaviours will promote recovery (early mobilisation) and cognitive coping training (thoughts and worries a patient has and ways to manage them) (Hagger-Holt 2009).
Pharmacology
Analgesia can be defined as relief from pain, thus analgesics are medicines that relieve pain. How much relief an analgesic provides results from the cause and nature of the pain, a patient’s previous pain history, gender and ethnicity (Cox 2010a).
Pharmacology describes the analgesic’s action and its interaction with the patient. It has four main divisions:
- pharmacodynamics – what the analgesic does to the body (e.g. reduces inflammation by inhibiting enzyme production)
- pharmacokinetics – what the body does to the analgesic (how the medicine is absorbed, distributed, metabolised and excreted)
- pharmacoeconomics – the cost and benefit ratio compared to other analgesics
- pharmacovigilance – how safe is the analgesic?
Perioperative Analgesic Medicines
Information on all licensed medicines can be found in a document called the Summary of Product Characteristics (SPC). The most up-to-date SPC can be found in the electronic Medicines Compendium (eMC) and downloaded at www.medicines.org.uk. All licence and dose information that follows has been provided from this source unless otherwise indicated.
Paracetamol
Paracetamol is one of the world’s most commonly used analgesics. It is understood that this centrally acting analgesic interacts with the cyclo-oxygenase (COX) system, endogenous opioids and serotonergic inhibitory pathways (Mattia and Coluzzi 2009). The oral formulation is well tolerated and very cheap. One gram of paracetamol in combination with codeine 60 mg produces greater analgesia than paracetamol alone. The intravenous formulation is now widely used during and after surgery (ANZCA 2010). A lower dose than the recommended 4 g daily maximum should be used in patients who have renal impairment.
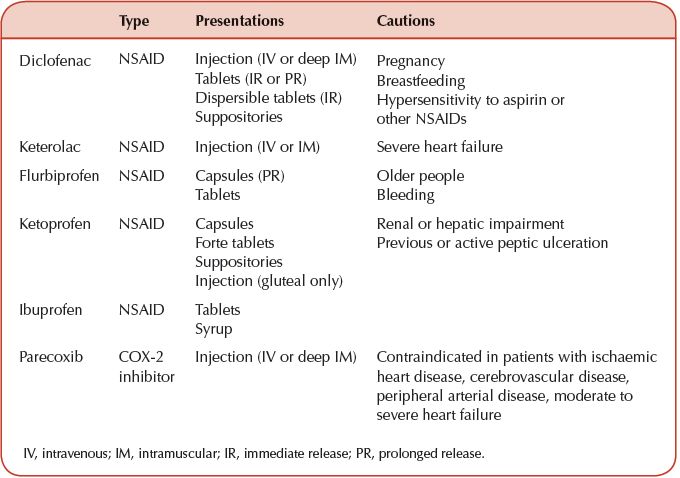
Table 24.2 Non-steroidal anti-inflammatory drugs (NSAIDs) and cyclo-oxygenase inhibitors (COX-2) properties for acute pain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


