Ionising radiation risks
Ionising radiation can cause biological damage to tissues. Damage is at a cellular level and can be minor and imperceptible, posing no long-term risk. It can also result in more serious biological damage; ionising radiation can trigger abnormal cell growth or differentiation of tissue, leading to cancer. Essential safety measures are required to regulate the medical use of radiation. The Ionising Radiation Regulations 1999 (IRR99) (Health and Safety Executive 1999) and the Ionising Radiation (Medical Exposure) Regulations 2000 (Department of Health 2000) provide clear legal requirements and controls on how ionising radiation is handled and administered in the UK.
Biological damage from ionising radiation can be classified by the potential effect it may have on tissue; the effect can be described using the following terms: somatic and genetic, deterministic and stochastic (ICRP 2007). A somatic effect is restricted to the individual who has received the dose of radiation. Cancer and cataracts can be induced by ionising radiation and are examples of a somatic effect. Genetic effects are caused by irradiation to the gonads, causing damage to the DNA in the sperm or ovum. Genetic effects do not appear in the person receiving the radiation dose; instead the effects are passed on to their descendants.
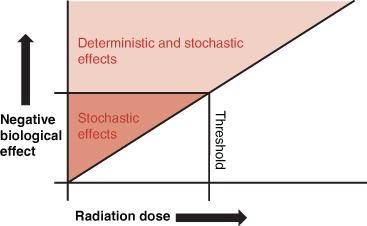
The potential for a biological effect is partly dependent upon the amount of the ionising radiation exposure (Figure 21.2). At small levels of exposure, the risk of developing a somatic or genetic effect is tiny, random and unpredictable; this is described as a stochastic effect. The probability of a stochastic effect increases with higher radiation doses. Stochastic effects are not immediate; they manifest later and cannot be directly attributed to a specific radiation exposure. When an individual is exposed to very high levels of ionising radiation beyond a known level or threshold, the resultant biological effects can be predicted or determined. These are deterministic effects and are normally only associated with therapeutic applications of ionising radiation and not diagnostic procedures. An example of a deterministic effect might be radiation-induced burns or erythema affecting the skin caused by a radiotherapy treatment.
There is a small probability that damage can occur with even the smallest exposure to ionising radiation (e.g. a chest X-ray). There is therefore no such thing as a safe dose of ionising radiation.
Ionising radiation, tissue sensitivity and pregnancy
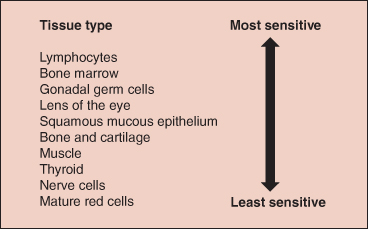
Certain tissues are particularly susceptible to biological damage when exposed to ionising radiation and can be described as radiosensitive. Generally tissues that have a high rate of cell division or differentiation are the most radiosensitive (Ball et al. 2008). Figure 21.3 demonstrates the radiosensitivy of various tissues.
When X-raying a pregnant woman the potential harm to the unborn fetus must be carefully considered and justified using the risk versus benefit principle. The fetus is particularly radiosensitive during the first trimester due to organogenesis. Depending upon the examination, the radiographer may need to confirm the pregnancy status of female patients of childbearing age before administering radiation.
Staff working with radiation should also be aware of the risks (Box 21.1).
What is radioactivity?
Radioactivity is a naturally occurring process where a substance composed of unstable atomic nuclei emits ionising radiation as the nuclei decay. As a nucleus decays it can emit three different types of radioactivity: an alpha particle, a beta particle or a gamma ray; all are ionising. Working with a radioactive substance is very different from man-made X-rays. In conventional radiography or CT, X-radiation is only produced when required. However, a radioactive source keeps emitting radiation until all the nuclei have decayed. The time it takes a source to decay is determined by its half-life, defined as the time taken to lose half of its radioactivity. The rate of decay cannot be stopped or altered but is determined by the type of radionuclide administered.
Table 21.1 Examples of radionuclides used in medical imaging.
From Watson et al. (2005).
| Radionuclide | Half-life | Clinical uses |
| Technetium-99 m | 6.02 hours | Numerous |
| Iodine-123 | 13 hours | Thyroid |
| Xenon-133 | 5.27 days | Lung ventilation |
| Thallium-201 | 3.1 hours | Heart muscle |
| Selenium-75 | 118.5 days | Adrenal glands |
Radioactivity and radionuclide imaging
In RNI (or nuclear medicine), radionuclides are manufactured specifically for medical imaging purposes and ideally emit gamma rays that can escape the body rather than alpha or beta particles that cannot. A radionuclide is combined with another pharmaceutical to form a radiopharmaceutical that is administered to the patient, enabling functional imaging of disease. RNI imaging techniques are used to complement the more anatomical diagnostic information gained from other medical imaging modalities such as CT and MRI. Table 21.1 lists some of the commonly used radionuclides in nuclear medicine.
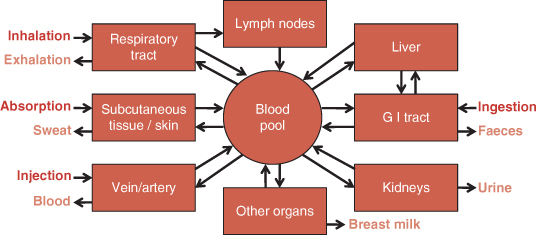
Radiopharmaceuticals can be administered into the body by several delivery methods (Figure 21.4). The patient then becomes an actual source of radioactivity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree