History
The patient was a 65-year-old retired man seeking treatment for dilated cardiomyopathy with normal coronary arteries for about 10 years. Risk factors are dyslipidemia and obesity (91 kg, 173 cm). Two procedures of ablation for atrial fibrillation and atrial tachycardia were performed 6 and 2 years earlier. One year previously, a new episode of atrial fibrillation was treated with direct current shock and amiodarone. The patient is currently in sinus rhythm and remains in New York Heart Association (NYHA) class III under medical therapy. The patient has no pulmonary disease, and respiratory tests are normal.
Comments
This is a long history of heart failure in a patient being followed adequately and benefiting from currently available therapeutic techniques. The cardiomyopathy is considered idiopathic, and atrial arrhythmias are events that do not fully explain the current heart failure status of the patient.
Medications
The patient was taking bisoprolol 2.5 mg daily, ramipril 5 mg daily, rosuvastatin 20 mg daily, furosemide 40 mg daily, warfarin with international normalized ratio (INR) between 2 and 3, amiodarone 200 mg daily.
Comments
The patient could not tolerate the recommended dosages of beta blockers and angiotensin-converting enzyme (ACE) inhibitors because of symptomatic hypotension. The spironolactone that was given previously had to be stopped for occurrence of hyperkalemia. Thus medical treatment is not optimal, because patient ideally should require 10 mg daily each of bisoprolol and ramipril. The statin is given for the hypercholesterolemia. Amiodarone and warfarin are for rhythm control and prevention of thromboembolism, respectively. The furosemide dosage is sufficient for controlling the heart failure symptoms.
Current Symptoms
The patient is in NYHA class III, with predominant dyspnea on exertion, accompanied with palpitations, fatigue, and mild peripheral edema.
Comments
The patient never experienced symptoms of acute heart failure even during the occurrence of atrial tachyarrhythmias. Increasing the dosage of furosemide did not minimize symptoms.
Physical Examination
Comments
Although the patient had symptoms of heart failure, no objective sign can be observed except from sinus tachycardia and hypotension. This may occur in patients with chronic heart failure.
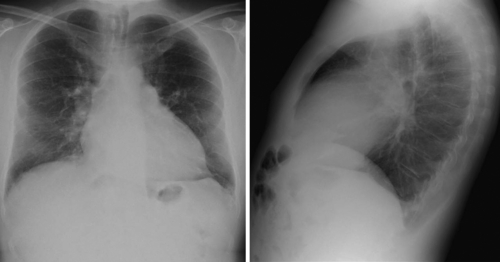
Laboratory Data
Comments
The patient has mild impairment of renal function, and B-type natriuretic peptide (BNP) is elevated.
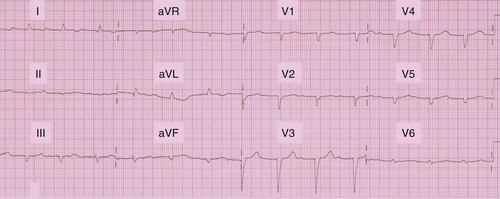
Electrocardiogram
Findings
The electrocardiogram (ECG) showed sinus rhythm 82 bpm, P duration 80 ms, P axis 40 degrees, PR interval 320 ms, QRS 120 ms, and QRS axis −30 degrees; QTc 489 ms (Figure 18-1).
Comments
The ECG showed first-degree atrioventricular block, and the QRS was not prolonged. QRS morphology could correspond to left anterior hemiblock, although left axis deviation is not that important. This ECG tracing does not fit a cardiac resynchronization therapy (CRT) indication.
Echocardiogram
Findings
The echocardiogram revealed left ventricular ejection fraction (LVEF) 25%; moderate left ventricular dilation, with end-diastolic volume 87 mL/m² and end systolic volume 65 mL/m²; global hypokinesia; left ventricular filling time less than 40% of the RR duration; left ventricular preejection time 160 ms; normal right ventricular function; dilation of the atria; no valvular abnormality; and increased filling pressures (Figure 18-2).
Comments
A discrepancy was seen between low LVEF and moderate left ventricular dilation, although the patient’s heart failure history is 10 years. The short left ventricular filling time is related to the prolonged PR interval. The prolonged preejection time may be related to left ventricular dyssynchrony. Increased filling pressure is compatible with the high BNP level.
Catheterization
A coronary angiogram was performed again (the first since 2006) to exclude coronary artery disease. The examination provides an opportunity to assess the coronary sinus network during venous-phase recording. This examination shows a huge distribution of very small and tortuous veins that predict major difficulties in case of a decision for a CRT implantation.
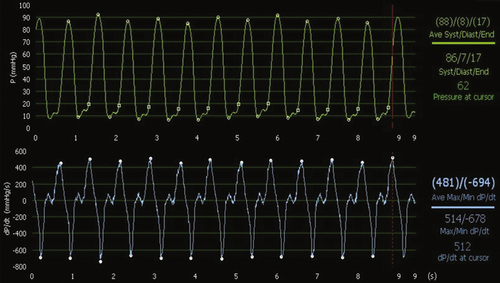
Analysis of the effects of atriobiventricular pacing with an endocardial left ventricular lead placed at different sites within the left ventricle is performed, and three temporary leads are introduced: one at the atrial appendage and one at the right ventricular apex via the right femoral vein and one left ventricular lead introduced via the right femoral artery from the groin. A radial approach was used for the coronary angiogram, to introduce a pressure wire to record left ventricular pressure, and to calculate left ventricular dP/dt. All pacing configurations are applied in the VDD mode, with the AVD set at 100 ms. Three left ventricular lead positions are tested in biventricular pacing: apical, anterolateral, and posterior. Each pacing is applied for 2 minutes before a 10-second recording to measure mean dP/dtmax and dP/dtmin. Between each pacing configuration, the baseline intrinsic rhythm is restored (Figures 18-3, 18-4, 18-5, and 18-6).
In conclusion, both dP/dtmax and dP/dtmin increase during biventricular pacing at all left ventricular sites in contrast to baseline spontaneous rhythm. However, the left ventricular pacing site does influence the result—when the left ventricular lead is placed at the left ventricular apex, the improvement in dP/dtmax is 7% (not significant), whereas it is 45% at the anterolateral left ventricular site, and 67% at the midposterior left ventricular site.
Comments
Left ventricular dP/dtmax is considered a hemodynamic reference parameter to assess cardiac function. However, the methodology of measurement is quite important, especially when differences in the effect of two therapies are small. Each measurement done under each pacing configuration must be compared to baseline applied between each pacing configuration. The different methods of measurement are not standardized. Measurements can be done after 30 seconds to 2 minutes in each pacing mode. Plateaus of several seconds or cycles are then compared, with the condition that no extrasystole occurs that would induce a drop in dP/dtmax. Another technique is to record a few cycles before and after the pacing configuration transition at a moment when the direct mechanical effect of the pacing configuration change is observed without peripheral adaptation. In any case, repetition of measurements should be performed to minimize the effects of artifacts, such as respiration, especially when observed differences between pacing modes are small. In the example, the biventricular pacing configuration with the left ventricular lead placed at the apex does not provide a significant difference in contrast to baseline AAI mode. However, the two other left ventricular locations did provide a significant improvement in dP/dt well above two times the standard deviation. A limitation in this case is the absence of repetition of dP/dt measurements in each pacing mode.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


