Fig. 3.1
From the National Heart, Lung, and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE), the Diamond probability of coronary artery disease (open bars) compared with actual observed coronary disease prevalence in symptomatic women (solid bars). Atyp Ang atypical angina, Nonang non-angina, Typ Ang typical angina (Reprinted from “Shaw et al. [14] with permission from Elsevier)
Symptoms Reported by Women
While both men and women will frequently report symptoms such as chest pain or pressure, symptoms reported by women also frequently fall into the classification of atypical angina or non-angina pain rather than typical angina. This can make it challenging for clinicians to accurately estimate their pretest probability of heart disease. It is important to have an appreciation for the variation in angina symptoms between men and women. Women are more likely to have angina at rest, during sleep, or with emotional or mental stress. They are also more likely to have symptoms such as neck and shoulder pain, nausea, vomiting, fatigue or dyspnea during an acute myocardial infarction [16].
Research on gender differences in symptoms has highlighted distinct differences in disease presentation. An observational study [17] of over one million patients with myocardial infarction demonstrated that while 70 % of men complained of chest pain, only 58 % of women complained of chest pain. Interestingly, this difference was most pronounced in younger women, and was not statistically significant in older women. While chest pain is certainly present in the majority of women with acute myocardial infarction, one study [18] demonstrated high rates of other symptoms including shortness of breath (58 %), weakness (55 %), and fatigue (43 %). Non-chest pain symptoms during the prodromal time leading up to a myocardial infarction were also frequently experienced. Notably, unusual fatigue (70 %), sleep disturbance (48 %), and shortness of breath (42 %) were more prevalent than chest discomfort (30 %). While actual symptom rates vary throughout the literature, the variety of non-chest pain symptoms in women remains impressive. A list of many of these common “atypical symptoms” is included in the Table 3.1 [10, 18–20].
Table 3.1
Variety of symptoms reported by women during ACS
Chest pain |
Neck/jaw/tooth pain |
Arm/shoulder/back pain |
Cold sweat |
Hot/flushed |
Fatigue |
Weakness |
Cough |
Heart racing/palpitations |
Shortness of breath |
Loss of appetite |
Indigestion/nausea/vomiting |
Arm numbness or burning |
Dizziness/lightheadedness |
Vision change |
Headache |
Awareness of the variety and frequency of symptoms other than chest pain is important for patients so that they might seek medical attention in a timely manner. Early recognition and treatment of an acute coronary syndrome can clearly improve outcomes, but disturbingly a recent review of emergency medical services found that only 23 % of patients call 9-1-1 when experiencing an acute coronary syndrome [21]. It is also important for clinicians to recognize symptoms so that they can appropriately diagnose and treat women presenting with cardiac ischemia. While a history of typical angina symptoms is frequently associated with obstructive CAD in both men and women, a history of atypical angina in a woman with the aforementioned symptoms should also warrant careful consideration by the clinician. Even in the absence of obstructive CAD, advances in diagnostic technology are increasingly identifying evidence of cardiac ischemia in symptomatic patients. This recognition of a pathophysiology for cardiac ischemia other than obstructive CAD could help to bridge the gap between lower rates of obstructive CAD in women with their high rate of poor cardiovascular outcomes. It is still unclear whether differences in the rates of underlying cardiac pathophysiology can help to explain some of the differences in symptoms experienced between the sexes.
Figure 3.2 reproduces the disparity between the lower incidence of myocardial infarction and worse outcomes in women compared to men [22].
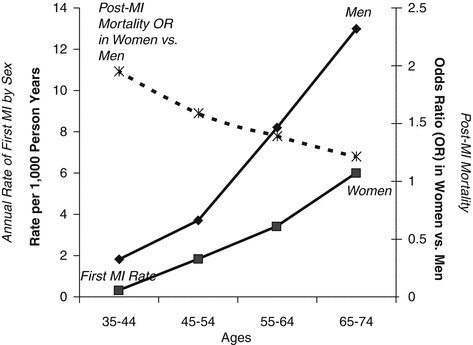

Fig. 3.2
Disparity between the lower incidence of myocardial infarction and worse outcomes in women compared to men (Reprinted from Merz. et al. [22] with permission from Elsevier)
Pathophysiology of Ischemic Heart Disease
As mentioned in the introduction, there is a gender paradox in that women appear to have less obstructive CAD and yet worse cardiovascular outcomes compared to men. In order to solve this gender paradox, multiple pathologies in addition to obstructive coronary disease have been suggested as contributing to IHD. These include obstructive CAD, plaque morphology, microvascular dysfunction, endothelial dysfunction, inflammatory conditions, and hormonal influence as displayed in Fig. 3.3.
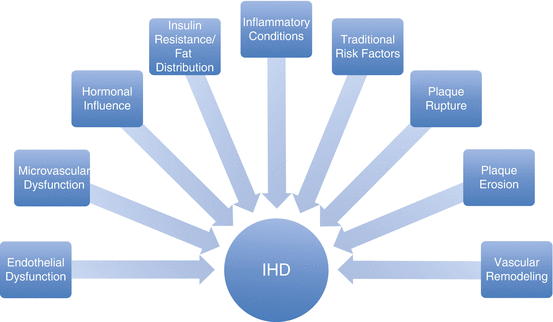

Fig. 3.3
Multiple factors contributing to Ischemic Heart Disease (IHD) in women
Obstructive Coronary Artery Disease
The heart is a muscular organ that receives its blood supply from the coronary arteries originating at the aorta. These coronaries run along the epicardium before feeding deep into the myocardium. The majority of coronary artery disease in both men and women arises from obstructive disease of the epicardial portion of these coronary arteries. Without adequate blood flow from the coronary arteries, myocardial tissue becomes ischemic and loses its ability to efficiently contract and conduct electrical signals. These vital epicardial arteries branch out from the aorta in similar patterns in both men and women, but women commonly have smaller coronary artery diameter and less collateral arteries branching off of the major epicardial coronary arteries than do men [23]. Obstruction of the coronary arteries is most attributable to the chronic accumulation of lipids in arterial walls by the process of atherosclerosis. Obstructive atherosclerosis is more commonly seen in men than premenopausal women, but post menopausal women develop obstructive atherosclerosis at rates similar to those seen in men [24]. Major risk factors for the development of atherosclerosis include hyperlipidemia, hypertension, tobacco abuse, and diabetes mellitus. On a cellular level, in atherosclerosis, LDL deposited in the vessel walls triggers endothelial cells to signal monocytes. These monocytes differentiate into macrophages, which cross the endothelial wall and phagocytize the LDL particles. They then become lipid-laden foam cells that trigger smooth muscle cells to accumulate and develop a surrounding fibrous cap. With degradation caused by inflammatory cells, the fibrous cap can rupture, which uncovers the thrombogenic lipid core. This triggers thrombus generation, which can cause obstruction of blood flow through the artery and myocardial ischemia. Acute plaque rupture as well as plaque erosion and chronic narrowing from atherosclerosis can be seen on coronary angiography by injecting contrast dye and visualizing disruption in flow through the epicardial coronary arteries [25–28].
Plaque Erosion vs Plaque Rupture
Autopsies performed on women who died from sudden cardiac death reveal gender differences in plaque morphology. Lipid laden plaques with a necrotic core are more prevalent in men and, as discussed above, will typically rupture leading to sudden exposure of inflammatory cytokines and thrombus formation at the site of the plaque. Conversely, young women in particular have higher rates of plaques composed of smooth muscle and proteoglycan-rich matrix without a necrotic lipid laden core. In contrast to plaque rupture, these plaques without a necrotic core exhibit superficial erosion leading to thrombus formation. With plaque erosion, the core of the plaque remains intact and thrombi form after coming in contact with smooth muscle. Autopsies reveal thrombi at the site of plaque erosion as well as distal embolization of thrombi formed from plaque erosion [5, 29–32]. Importantly, these gender differences in plaque morphology tend to be most pronounced in younger pre-menopausal women whereas post-menopausal women generally have fewer differences in plaque morphology and incidence of obstructive CAD.
Remodeling
In response to atherosclerosis, coronary arteries undergo remodeling to preserve adequate blood flow. When plaques involve less than 40 % of the luminal area, “positive” or “outward” remodeling can lead to enlargement of the artery which preserves the intraluminal cross sectional area and blood flow [33]. Conversely, with “negative” or “inward” remodeling, the cross sectional area of the vessel is reduced and flow limitation is seen on coronary angiography. Whether a specific vessel will exhibit positive or negative remodeling continues to be an area of research, but some factors such as increased proteases from inflammatory cells seem to correlate with more positive remodeling [31]. Authors have suggested increased amounts of positive remodeling in women compared to negative remodeling in men as a way to reconcile the more diffuse atherosclerosis, increased endothelial dysfunction, and increased microvascular dysfunction seen in women with the decreased amount of luminal obstruction observed on coronary angiography compared to men [14, 29, 31, 34].
Microvascular Angina
In contrast to angina secondary to obstruction of the large epicardial coronary arteries, microvascular angina (previously referred to as cardiac syndrome X) is a term used to describe angina symptoms from ischemia originating in intramyocardial microvascular arteries. As mentioned in the introduction of this chapter, women with chest pain presenting for angiography more frequently lack obstructive coronary artery disease than men presenting for angiography [6–8], suggesting that there is a pathology other than obstructive coronary disease responsible for their symptoms. While the pathophysiology of microvascular angina is an ongoing topic of research, two of the major contributing factors appear to be endothelial-independent microvascular dysfunction and endothelial-dependent dysfunction [5]. This microvascular angina has been postulated to be more prevalent in women because of higher levels of inflammation and hormonal changes throughout women’s lives. The higher prevalence of microvascular angina in women compared to men has led authors to address it primarily as a disease of women’s hearts [35].
Microvascular Dysfunction
Microvascular dysfunction refers to disease in small coronary resistance vessels measuring 100–200 μm. It can encompass the abnormal coronary reactivity that is attributable to distal embolization from coronary plaque erosion, smaller arterial size, and positive remodeling [22, 29]. In the WISE study, one subset of 159 women with angina and non-obstructive CAD on coronary angiography underwent coronary flow reserve (CFR) testing. CFR was tested by injecting intracoronary adenosine and measuring the coronary velocity response. The CFR was found to be decreased in 47 % of these women, indicating disease of the microvasculature even in the absence of obstructive CAD [29]. Nuclear Magnetic Resonance Spectroscopy (NMRS) is a non-invasive imaging technique that uses a probe to detect different quantities of chemicals in a sample. Cardiac NMRS was also used in the WISE study to look for myocardial ischemia by measuring phosphocreatine/adenosine triphosphate ratio during handgrip exercise. Decrease in the phosphocreatine/adenosine triphosphate ratio in the heart signifies a shift from aerobic to anaerobic cellular metabolism, which indicates myocardial ischemia. In WISE, women with angina but no obstructive CAD, 20 % still showed evidence of myocardial ischemia on NMRS when performing handgrip exercise [8, 9, 36].
Retinal arteriolar narrowing is a noninvasive peripheral measure that can also be used to evaluate microvascular disease throughout the body [37]. In women with retinal arteriolar narrowing, there is an increased risk for development of IHD. In contrast, men with retinal arteriolar narrowing do not appear to have a significantly increased risk of developing IHD. Together, this combination of functional and pathological findings demonstrate that there are differences in the vasculature in men and women and suggests a significant role for the microvasculature in IHD. While obstructive CAD is established as the predominant pathology in men, microvascular disease appears to contribute disproportionately to the pathophysiology of heart disease in women.
Endothelial Dysfunction
Coronary endothelial function plays a role in regulating myocardial blood flow and can also be a predictor for future vascular disease. In the WISE study, investigators measured the change in coronary flow reserve in response to injections of acetylcholine (activating endothelial-dependent dilation), adenosine (non-endothelial-dependent microvascular dilation), and nitroglycerine (non-endothelial-dependent epicardial dilation) in women with angina but no obstructive CAD [38]. They identified that women with a poor response to acetylcholine had a higher rate of adverse IHD outcomes over a 4-year follow up. This suggests that endothelial dysfunction independently predicted and likely contributed to IHD. This warrants further research and may indicate a potentially new therapeutic target. In addition to invasive intracoronary injection of acetylcholine, brachial artery flow mediated dilation has been used as a peripheral measure of endothelial function and has also been correlated with increased IHD risk in women [5].
Risk Factors
Risk factors for microvascular dysfunction and endothelial dysfunction include many of the traditional risk factors associated with coronary atherosclerotic disease including obesity, dyslipidemia, hypertension, diabetes, and smoking. Increasing evidence also identifies psychological factors including stress and depression as having significant association with IHD and acute myocardial infarction [9, 39–42]. In women, and especially in post-menopausal women there is an increased clustering of many of the traditional risk factors for heart disease [5]. Additionally, vasculitis and general inflammatory auto-immune diseases such as lupus, rheumatoid arthritis, and thyroiditis have been associated with cardiac disease [14, 32]. Many autoimmune diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) have female predominance. Women with lupus in particular have been shown to have higher rates of cardiac disease beyond what would be estimated by their baseline traditional risk factors [43]. Accelerated atherosclerosis in patients with SLE and RA is associated with increased cardiovascular morbidity and mortality [44]. Inflammatory conditions are also more prevalent in women and general rates of inflammation as reflected by elevated C-reactive protein have been shown to be elevated in women [14]. The combination of risk factor clustering, higher rates of depression, higher rates of vasculitis, and higher rates of general inflammatory conditions could offer some explanation for the disproportionate burden of heart disease and specifically higher rates of microvascular and endothelial dysfunction in women.
Hormonal Influence
Women also have an element of endothelial dysfunction that appears to correlate with hormonal changes throughout their lives and the lack of positive estrogenic effects on blood vessels after menopause [5, 42]. Estrogen has been identified as a likely factor contributing to the lower risk of IHD in premenopausal women compared to age matched male controls. After menopause, the reduction in estrogen appears to be accompanied by a decrease in its protective effects and an increase in the risk of IHD. Estrogen in the female heart appears to have a number of beneficial effects, which notably include improved endothelial function and decreased flow resistance as well as improved vascular response to injury. Experimental evidence points to increases in nitric oxide production and up regulation of nitric oxide genes as a mechanism by which estrogen improves vasodilation. Estrogenic effects on Estrogen Receptors (ER) alpha and ER beta in the vasculature appear to play a major beneficial role in flow resistance and vascular response to injury.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


