Agent
Class
Gender-specific variable analysed
Gender-related variances
References
Antiplatelets
Aspirin
COX-1 inhibitor
Platelet reactivity in women treated with aspirin.
Higher agonist-induced platelet reactivity in women
Becker et al. [9]
Pharmacokinetic properties
Quicker absorption and larger distribution volume in women
Buchanan et al. [10]
Drug bioavailability
Slower drug clearance in females
Ho et al. [11]
Clinical outcome for primary and secondary prevention in females versus male patients
No difference observed in meta-analysis of clinical trials
Woodfield et al. [12]
Baigent et al. [13]
P2Y12 receptor inhibitors
Clopidogrel
Thienopyridine
Platelet function inhibition by clopidogrel
No gender-related differences
Ferreiro et al. [14]
Reduction of the combined risk of death, MI or stroke at one year
Greater risk reduction among women
Steinhubl et al. [15]
Meta-analysis of five large phase III clinical trials focused on sex-related differences in clinical outcome
No statistical significant heterogenicity with regard to gender
Berger et al. [16]
Prasugrel
Thienopyridine
Reduction in ischemic events comparing prasugrel with clopidogrel
No interactions between treatment effect, bleeding and gender
Wiviott et al. [17]
Ticagrelor
Cyclopentyl-triazolopyrimidine
Composite ischemic event rate comparing ticagrelor with clopidogrel
No interactions between treatment effect, bleeding and gender
Wallentin et al. [18]
GPI
Abciximab
GP IIb/IIIa antagonist
In vitro platelet response to abciximab
No gender-related differences
Coller et al. [19]
Meta-analysis of large clinical trials with regard to major adverse events
No differences with regard to adverse outcome, higher major and minor bleeding rates in women
Cho et al. [20]
Eptifibatide
GP IIb/IIIa antagonist
Composite adverse clinical events and bleeding rate
No differences in death, MI or target vessel revascularization, higher major and minor bleeding rates in women
Fernandes et al. [21]
Tirofiban
GP IIb/IIIa antagonist
Biomarker in ACS
CRP and brain natriuretic peptide more likely elevated in women than men
Wiviott et al. [22]
Anticoagulants
Heparins
UFH
Indirect factor Xa and Thrombin inhibitor
aPTT values
Higher in women
Granger et al. [23]
LWMH
Indirect factor Xa and Thrombin inhibitor
Reduction in the composite of death and MI
Larger reduction of the primary endpoint and more minor bleedings in female patients
FRICS study group [24]
Meta-analysis of phase III clinical trials focused on sex-related differences in clinical outcome
No gender-related interactions with regard to triple endpoint, death or MI significantly reduced in women but not men
Toss et al. [25]
Clinical adverse outcome in fibrinolysis plus heparins
No gender-related interactions
Cohen et al. [26]
Mega et al. [27]
Warfarin
Vitamin K antagonist
Risk of thromboembolism under treatment with a vitamin K antagonist
Higher benefit in women
Fang et al. [28]
Major bleeding risk
No gender-related interactions
Hughes et al. [29]
Bivalirudin
Direct factor IIa inhibitor
Ischemic outcomes and major bleeding rates under treatment with bivalirudin plus provisional use of GPI
Female gender significantly associated with major bleeding risk
Chacko et al. [30]
Ischemic outcomes and bleeding rates under monotherapy with bivalirudin
No gender-related differences with regard to ischemic outcomes
Manoukian et al. [31]
No gender-related differences
Mehran et al. [32]
Dabigatran
Oral direct factor IIa inhibitor
Thromboembolic events and bleeding risk in patients with atrial fibrillation
No gender-related interactions, safe and effective in women and men
Connolly et al. [33]
Fondaparinux
Indirect factor Xa inhibitor
Reduction in the composite endpoint of death, MI, refractory ischemia or major bleeding
Higher absolute reduction of major bleeding in women with a trend for statistical interaction
Reduction in the composite of death or re-infarction
No gender-related interactions
Oldgren et al. [36]
Rivaroxaban
Oral direct factor Xa inhibitor
Thromboembolic events and bleeding risk in patients with atrial fibrillation
No gender-related interactions, safe and effective in women and men
Patel et al. [37]
Apixaban
Oral direct factor Xa inhibitor
Thromboembolic events and bleeding risk in patients with atrial fibrillation
No gender-related interactions, safe and effective in women and men
Granger et al. [38]
Definitions, Terms and Classifications
Epidemiology of Thrombotic Disorders in Women
Atherothrombotic disorders (ischemic heart disease and stroke) cause approximately a quarter of the total women mortality worldwide [39]. Coronary artery disease (CAD), just in the United States, accounted for 15.6 % of deaths in women in 2007, and cerebrovascular disease (CVD) accounted for another 6.7 % [40]. In 2007, among the 607,000 American women hospitalized for CAD approximately one million underwent cardiac catheterization, percutaneous coronary intervention (PCI), stenting, or coronary artery bypass graft procedures. The average length of hospital stay was 5.3 days for acute myocardial infarction (MI) alone. The same year, 458,000 women were hospitalized for CVD, with an average hospital stay of 5.4 days [41].
Underestimation of cardiac risk and misconception of symptoms of CAD in women usually lead to less referral for cardiac testing, appropriate treatment and coronary catheterization during ACS [42–44]. Moreover, women used to be older than men when they present CAD and thus excluded more frequently from randomized studies [45, 46]. The prevalence of CAD is higher among men until the age of 39 (15.9 % vs. 7.8 %, respectively), although in the range 40–59 years (37.9 % and 38.5 %) and in the range 60–79 years (73.3 % and 72.6 %) the prevalence is very similar [47]. On the contrary, after 80 years of age women have more CAD than men [48]. Therefore, the onset of clinical manifestations of CAD occurs about 10 years later in women versus men [42]. Figure 15.1 shows how as women become older the incidence of CAD, stroke, and peripheral artery disease increases considerably [5]. Essentially, around the time of menopause, the incidence of CAD more than triples [40]. Additionally, there are growing proportions of costs of CAD and CVD, estimated at $505 billion in the United States alone [49], given the longer life expectancy of women relative to men worldwide [38].
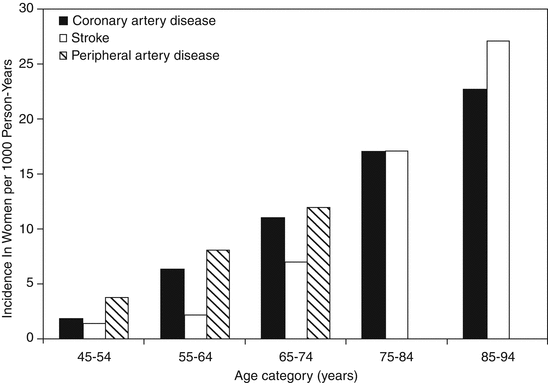

Fig. 15.1
Incidence of atherothrombotic disease in women by age category. Data for coronary artery disease and stroke are from the Framingham original and offspring cohort, and data on peripheral arterial disease are from the ARIC (atherosclerosis risk in communities) cohort (per 1,000 person-years) (Reprinted with permission Wang et al. [5])
Currently, the rate of recurrent MI and cardiovascular death among women has increased compared with men, despite the decrease of CAD mortality among men because of the advances in the diagnosis and treatment of ACS [50, 51]. Even though women present a higher risk profile compared with men, the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines? (CRUSADE) National Quality Improvement Initiative showed that ACS women less often receive guideline-recommended antithrombotic therapies [42].
Gender Disparities in Platelet Biology
Platelet Reactivity
There are described differences in platelet reactivity across populations using several methods and in response to variable stimuli [52] (Fig. 15.2). Compared with platelets from men, those from women without CAD are more reactive in response to standard concentrations of agonists such as adenosine diphosphate (ADP) and thrombin receptor agonist protein [53, 54]. The total of glycoprotein (GP) IIb/IIIa receptors that can bind PAC-1 antibody in response to ADP or thrombin receptor agonist protein stimulation seems to be 50–80 % greater in women than in men [54]. Compared with men after adjustment for risk factors such as smoking, hypertension, diabetes, hyperlipidemia, and aspirin use, platelets from women, in particular, white women, bound more fibrinogen in response to low and high concentrations of ADP and showed more spontaneous aggregation among asymptomatic patients [55]. High platelet reactivity was related with an increased risk of downstream MI (two- to three-fold increased risk compared with those with normal platelet reactivity) in a population-based study of asymptomatic, premenopausal women [56]. Considerably higher levels of signaling cascade proteins expressed on platelets from male versus female donors were found in a recent study of signaling proteomes in platelets [57]. These initial findings suggest that sex-specific platelet proteomic signaling mechanisms could contribute to variances in platelet reactivity and outcome differences between women and men, even though this concept entails further investigation.
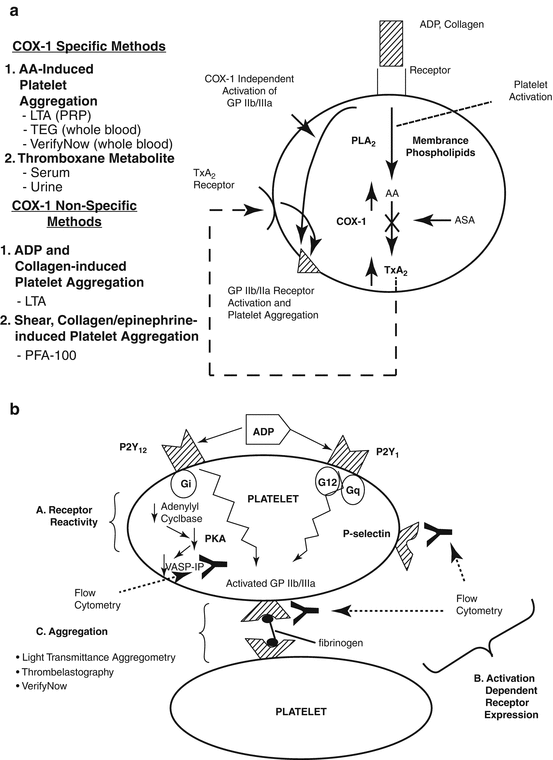

Fig. 15.2
Platelet responsiveness to aspirin and clopidogrel. Laboratory measurement of platelet responsiveness to aspirin (a) and clopidogrel (b). AA arachidonic acid, ADP adenosine diphosphate, ASA acetylsalicylic acid (aspirin), COX cyclooxygenase, GP glycoprotein, LTA light transmittance aggregometry, PFA platelet function analyzer, PKA protein kinase A, PLA2 phospholipase A2, PRP platelet-rich plasma, TEG thrombelastography, VASP vasodilator-stimulated phosphoprotein, Y monoclonal antibodies (Reprinted with permission Gurbel et al. [52])
Healthy men and women were also studied in the Genetic Study of Aspirin Responsiveness (GeneSTAR) study, which proved higher platelet reactivity among women compared with men in response to varying concentrations of arachidonic acid, ADP, or epinephrine after adjustment for age, risk factors, race, menopausal status, and hormone therapy [9]. Nevertheless, the study found comparable grades of platelet inhibition among men and women, in response to arachidonic acid after receiving a low-dose of aspirin. However, women’s platelets were more reactive than those of men in response to collagen or ADP stimulation after aspirin therapy, suggesting upregulation of platelet activation pathways indirectly related to cyclooxygenase-1 (COX-1) [9]. Moreover platelet-related gender differences, compared with men a greater prothrombotic tendency (augmented speed and strength of platelet-fibrin clotting) among women was also shown by thromboelastography-based studies [58, 59].
Inflammation
An inflammatory response mediated by activated platelets, releasing cytokines and immunomodulatory ligands, additionally intensifies platelet response and endothelial activation during plaque rupture [60]. Exposure of its core contents on acute plaque rupture, promotes adhesion of platelet receptors and integrins to von Willebrand factor and collagen in the subendothelium [61, 62]. Releasing of proinflammatory cytokines and inflammatory factors and binding to leukocytes to form platelet-leukocyte coaggregates are also facilitated by activated platelets, which may promote an inflammatory response within the vessel wall [62, 63]. In the Women’s Health Study, a higher C-reactive protein (CRP) level, the most frequently investigated marker of inflammation, independently predicted among healthy postmenopausal women the risk of cardiovascular death, nonfatal MI, stroke, or need for coronary revascularization (relative risk: 1.5 for the highest quartile versus the lowest; 95 % confidence interval [CI]: 1.1–2.1) [64].
A recent meta-analysis showed a risk ratio for CAD of 1.41 (95 % CI: 1.13–1.75) among women in five studies whose CRP level was 3.0 mg/l versus 1.0 mg/l, even though the Cardiovascular Health Study showed that CRP did not add to risk prediction for women but only for intermediate-risk men [65, 66]. There are other inflammatory markers, such as leukocyte count and P-selectin, which can be higher among healthy women and predictive of imminent cardiovascular events [67, 68]. Beyond their direct platelet-mediated effects, antiplatelet therapies have also been related with pleiotropic anti-inflammatory effects [69]. The synergy of aspirin in addition to statins in lowering CRP levels in a population-based, longitudinal stroke study in which 50 % of the participants were women did not vary by gender [70]. In one nonrandomized study, even after adjustment for gender, clopidogrel therapy was correlated with less level of inflammatory markers [71]. CRP levels increased 1 month after clopidogrel interruption without significant sex variances in another small study in which 50 % of the participants were women [72]. Regarding novel and more potent antithrombotic therapies, if these drugs can lead to selective improvements in clinical outcomes according to gender through a greater reduction in inflammation remains unknown.
Role of Hormones in Platelet Biology and Inflammation
Genomic effects in megakaryocytes, signaling properties in platelets, or both, might contribute to sex differences in platelet function given that megakaryocytes and platelets express the estrogen receptor [73] and androgen receptor [73, 74], and platelet nitric oxide synthase release and thromboxane A2 (TXA2) generation can be modulated by estrogens and/or androgens [74, 75]. Moreover, during megakaryocyte differentiation transcripts from both estrogen receptor and androgen are upregulated [74].
The evidence on the effect of female menstrual-cycle hormones on platelet biology is controversial. One study suggested that hormones may regulate platelet GP IIb/IIIa activation, showing that platelets bound more fibrinogen during the luteal phase than the follicular phase [55]. During the menstrual cycle, platelet adhesion to vascular collagen also shows biphasic peaks [73]. On the contrary, no significant relationship between platelet aggregability and menstrual cycle phase, oral contraceptive use, or menopausal state was found by other studies [57, 76, 77]. The expression of proteins in the coagulation-fibrinolytic pathways is altered by both oral contraceptive and perimenopausal hormonal agents, and may contribute to increased thrombotic risk promoting fibrinogen binding to platelets, increasing factor VII levels and activity, and levels of fibrinogen and plasminogen activator inhibitor-1 [78]. The relationship between hormones and atherothrombotic risk could be mediated also by the systemic inflammation. Among post-menopausal women treated with estrogen, randomized [79] and cross-sectional [80] studies have shown increased CRP levels. Nevertheless, baseline CRP did not found a significant statistical interaction with hormone therapy in predicting CAD risk in a nested case–control analysis from the two randomized, controlled studies of the Women’s Health Initiative [81].
A link between genetic polymorphisms for platelet glycoproteins and the risk of atherothrombotic events has been investigated in several studies [82–84], whether sex-specific differences influence the impact of these polymorphisms on platelet biology, treatment response, and patient outcomes remains still unknown. The HERS (Heart Estrogen–Progestin Study) showed that among women who received placebo women homozygous for the GP Ibα-5 T allele, heterozygotes and homozygotes for the GP Ibα-5C allele had a significantly lower incidence of the composite endpoint (death, MI, or unstable angina) at 5.8 years of follow-up [85]. In women with the -5C allele versus the -5TT genotype postmenopausal hormone treatment was associated with a 46 % lower adjusted cardiovascular risk (p < 0.001 for interaction) [85]. In women who had the GP Ibα-TT and GP VI-TC/CC genotypes (21.2 %) hormone therapy was associated with significant harm, but women with the GP IbαTC/CC and GP VI-TT genotypes (16.6 %) it was associated with significant benefit [85]. Thus, the risk-benefit ratio for proposed antiplatelet treatment may be altered by pharmacogenomic testing which can determine underlying cardiovascular risk in postmenopausal women. However, additional investigations are indeed warranted.
Sex Differences in Bleeding with Antithrombotic Therapy
Patients with ACS who receive antithrombotic drugs have shown to be associated with a fivefold increased risk of death at 30 days and a 1.5-fold increased risk of death between 30 days and 6 months [86–88]. Women randomized to receive aspirin for primary prevention of cardiovascular disease versus placebo in the Associated With Antiplatelet Therapies The Women’s Health Study, experienced more frequently serious gastrointestinal (GI) bleeding requiring transfusion (relative risk: 1.40; 95 % CI: 1.07–1.83) [89]. Nevertheless, 2.5 major bleeding events per 1,000 women versus three major bleeding events per 1,000 men after aspirin use over an average 6.4 years occurred according to a meta-analysis by Berger et al. [90]. There was no evidence of difference of effect between women and men for major bleeding (p < 0.24) for clopidogrel treatment, but the odds ratio for bleeding was numerically higher among women than men (1.43; 95 % CI: 1.15–1.79 vs. 1.22; 95 % CI: 1.05–1.42) [90]. In a multivariable risk model, Subherwal et al. consistently identified sex as an independent predictor of in-hospital major bleeding (odds ratio: 1.31; 95 % CI: 1.23–1.39) [91]. Additionally, women who underwent invasive treatment with PCI had significantly higher rates of in-hospital major bleeding compared with men (14.1 % vs. 5.9 %, p < 0.001) in the CRUSADE registry [92]. Despite GI bleeding after MI is more common in men, access-site complications are more frequent in women undergoing PCI (e.g., access site bleeding, retroperitoneal bleeding) [93, 94].
Other sex-specific differences, such as in vascular reactivity [95], could also contribute to differences in bleeding risk in addition to bleeding-site differences may relate in part to the smaller blood vessels in women [96]. Overall among women with less body mass, the question of weight-based dosing has been raised for antiplatelet agents such as clopidogrel [97], given that it could provide a mechanism for variations in antiplatelet response. Therefore, there was identified a significant interaction between sex, GP IIb/IIIa inhibitors (GPI) use, and bleeding risk (p < 0.014) in the CRUSADE registry (Fig. 15.3) [92]. Inappropriate dosing when using antithrombotic therapy is one of the main reasons of the higher bleeding risk in some women. In fact, an excess dosing of unfractionated heparin, low molecular weight heparin, or GPI could explained an estimated 15 % of the major bleeding showed in CRUSADE [98]. In fact, women remained at higher risk of excess dosing and bleeding than did men even after adjustment for age, weight, and renal function. Only 27 % of the risk in men is due to excess dosing of these agents while it reaches 72 % of the increased bleeding risk among women [92]. Compared with men, women receiving appropriately dosed bivalirudin had also higher rates of bleeding complications in the Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) [99] and Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trials [100]. In the setting of ACS there are also important implications of bleeding like a lower probability to be discharge on antiplatelet therapy [101], maybe because its reinitiation is postponed until the patient is considered “safe” from additional bleeding although this treatment “gap” can last up to 6 months after the initial event [101]. A higher probability to have a heightened systemic inflammatory response has also been described [102]. Importantly, the higher incidence of in-hospital bleeding among women has major implications for their risk of future ischemic events.
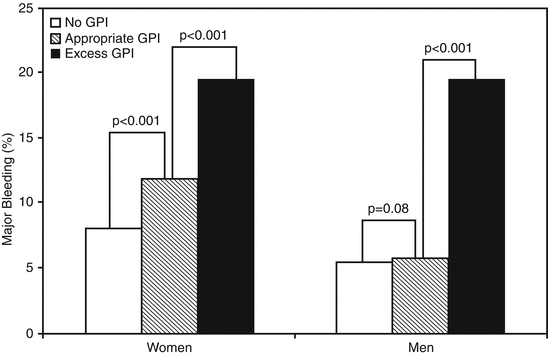

Fig. 15.3
Major bleeding by sex and GPI dosing. Incidence of in-hospital major bleeding among women and men with acute coronary syndromes who did not receive a GP IIb/IIIa inhibitor (GPI), those who received appropriate GPI dosing, and those who received excess GPI dosing in the CRUSADE registry. Probability values represent unadjusted comparisons (Adapted with permission from Alexander et al. [92])
AF, stroke, prosthetic heart valve replacement, MI and venous thrombosis are included among the indications of vitamin K antagonists (VKA). The main contributors which determine the bleeding risks in patients on oral anticoagulation have extensively been investigated [103]. In patients treated with VKA, patient characteristics, such as age and comorbidity, intensity of anticoagulant effects, length of therapy, and interaction with drugs that interfere with hemostasis are the determinants which primarily influence the risk of bleeding [14, 104]. However, female sex has not been described to be associated with major bleeding, including life-threatening bleeding events, such as intracranial hemorrhage [29]. However, in patients on oral anticoagulant therapy female sex appears to be an independent risk factor for minor bleeding [105, 106]. The association of sex and heparin-induced bleeding has not been consistently reported [107].
Antiplatelet Therapy
Gender-Related Differences in Response to Antiplatelet Drugs
Aspirin
Numerous studies have proved that aspirin reduces the risk of thrombotic events in patients with CAD [38, 108, 109]. In both sexes, aspirin inhibits similarly the formation of TXA2 synthesis in platelets by blocking selectively and irreversibly acetylating COX-1 [10, 11, 108]. However, in women pathways that are indirectly related to COX-1, such as those stimulated by collagen, ADP and epinephrine, are less inhibited compared with men [9]. Several ex vivo assays have shown that these differences persist after adjustment for several potential confounders, such as race, and results in higher platelet reactivity in aspirin-treated women with CAD [110–112]. A substudy of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) and the Heart Outcomes Prevention Evaluation (HOPE) trial showed higher risk of cardiovascular events among women. These trials found greater in vivo platelet activation related with female gender that was demonstrated to be a determinant of 11-dehydro thromboxane B2 concentration, a marker of aspirin resistance [113, 114].
Despite 6 randomized trials (95,456 patients) that were included in the aforementioned meta-analysis from Berger et al. showed that primary prevention with aspirin therapy significantly reduced the risk of cardiovascular events in both sexs [12 and 14 % reductions in women and men, respectively] [97], the benefit was primarily driven by a reduction of MI in men (odds ratio [OR] 0.68, 95 % confidence interval [CI] 0.54–0.86, p = 0.001) and ischemic stroke in the 51,342 women included (OR 0.76, 95 % CI 1.35–2.20, p < 0.001). Notably, as above mentioned, the risk of bleeding was significantly increased by aspirin to a similar degree in both sexes [97]. Tests for heterogeneity of treatment effect were not reported. Significant reductions in major coronary events and serious vascular events with aspirin therapy were found in men, but not women in both primary and secondary prevention studies from the ATT (Antithrombotic Trialists) Collaboration meta-analysis, that included the same studies but had access to individual participant data, estimating consequently in a more accurate way the magnitude of several risk factors, such as gender, in influencing selected outcomes [13]. Nevertheless, no heterogeneity of treatment effect was distinguished by sex for any of the endpoints evaluated after adjustment for multiple comparisons. These results suggested that the absolute risk reduction essentially depends on the individual’s absolute risk without treatment, given that the relative risk reduction among men and women is similar.
Aggregate data from 16 randomized trials of aspirin use in the secondary prevention setting, with stratification by sex were additionally reported (Fig. 15.4) [13]. Equally, the decrease in vascular mortality with aspirin versus placebo therapy after acute MI compared with men (22 %) was just 16 % (p < 0.05 for heterogeneity), in the International Study of Infarct Survival-2 (ISIS-2) trial [38]. In line with this, gender mix may be the cause of around a 25 % of the variation in the reported efficacy of aspirin in reducing the rates of cardiovascular events across placebo-controlled trials [115]. Accordingly, greater benefits of aspirin in decreasing non-fatal MI rates have been proved in trials primarily with men compared with those containing mostly women [115].
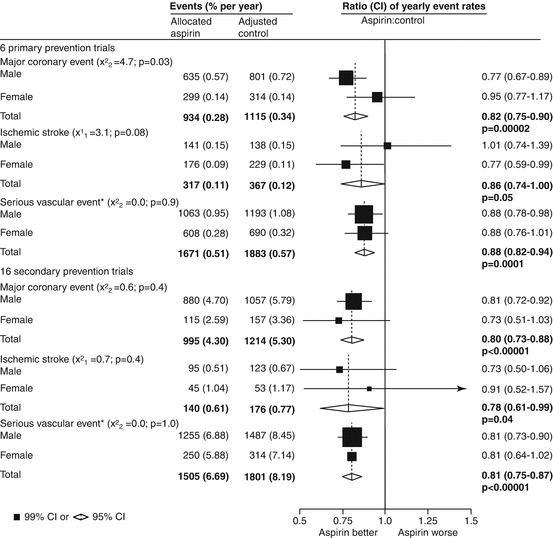

Fig. 15.4
Selected outcomes in primary and secondary prevention trials of aspirin, by sex. CI confidence interval (Reprinted with permission from the ATT (Antithrombotic Trialists) Collaboration Baigent et al. [13])
Compared with lower doses, no clear overall benefit with aspirin doses 100 mg was found in secondary analyses from previous clinical trials [116–118]. Additionally, no particular benefit related with a higher aspirin dose among women in these secondary studies was found, even though the higher platelet reactivity demonstrated in women [9, 53–55]. Moreover, in cases where the potential benefit compensates the potential risk of GI bleeding, the U.S. Preventive Services Task Force recommends low-dose aspirin for the prevention of MI among men age 45–79 years and for prevention of stroke among women age 55–79 years, according to sex differences in the epidemiology of cardiovascular disease and available evidence supporting therapy [119]. Likewise, for persons whose 10-year cardiovascular risk is at least 6 % aspirin therapy is recommended by the American Stroke Association and American Heart Association [120]. The 2007 update to the AHA guidelines for CVD prevention in women defined women at high risk as those with “established CHD, CVD, peripheral arterial disease, abdominal aortic aneurysm, end-stage or chronic renal disease, DM, and 10-year Framingham risk >20 %” [121]. The 2011 AHA guidelines for prevention of CVD in women recommended aspirin (75–325 mg/day) as a consideration in those who are at high risk regardless of age or for women >65 years of age who are at risk or healthy, depending on risk of hemorrhage and consideration of risk for ischemic stroke. These guidelines also recommended the use of specific risk prediction instruments that include CVD as part of global risk assessment [122–124].
Adenosine Diphosphate P2Y12 Receptor Antagonists
Thienopyridines inhibit irreversibly platelet activation blocking platelet ADP P2Y12 receptor. The first generation thienopyridine ticlopidine has been largely replaced in clinical practice by clopidogrel, a second generation thienopyridine because of its more favorable safety profile [125]. There is no difference between men and women in plasmatic levels of clopidogrel’s active metabolite [126]. Nevertheless, clopidogrel-induced inhibition of platelet aggregation presents some gender variability [127–129]. In patients presenting with unstable angina (UA)/non-ST elevation myocardial infarction (NSTEMI), the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial, demonstrated a smaller absolute (1.2 % vs. 2.8 %) and relative (12 % vs. 25 %) risk reduction in the composite endpoint of cardiovascular death, non-fatal MI or stroke at 1 year in women compared with men with clopidogrel plus aspirin compared to aspirin alone at 1 year [130], with similar findings in the subgroup of patients undergoing PCI [131]. On the contrary from the CURE trial, in patients undergoing elective PCI, the Clopidogrel for the Reduction of Events During Observation (CREDO) trial showed a 26.9 % relative risk reduction in favour of clopidogrel for the composite of death, MI and stroke at 1 year in the overall population, but demonstrated a greater risk reduction of the combined risk of death, MI or stroke at 1 year (32 % vs. 25 %) among women [15]. Patients treated with fibrinolytic therapy within 12 h after the beginning of STEMI symptoms in the CLARITY-TIMI 28 (Clopidogrel as Adjunctive Reperfusion Therapy – Thrombolysis in Myocardial Infarction 28), were randomized to dual antiplatelet therapy with aspirin plus clopidogrel versus clopidogrel alone. Despite a higher event rate among women in both treatment arms, a 36 % reduction in the risk of the composite ischemic endpoint with clopidogrel in the overall population, with similar reduction for men and women (35 % vs. 38 %, respectively) was observed [132]. In patients undergoing PCI 3 days after starting the assigned study medication the OR of the composite endpoint of cardiovascular death, recurrent MI or stroke at 30 days was 59 % in women and 41 % in men. The ClOpidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT) trial enrolled Chinese patients with suspected MI and compared the effect of clopidogrel plus aspirin versus aspirin alone. This trial demonstrated similar reductions in the primary ischemic endpoint at 28 days with no heterogeneity in effect related to sex, but with higher event rate in women [133]. Also in the CHARISMA trial, aforementioned, no clinical benefit was associated to the combination of a low-dose aspirin and clopidogrel in asymptomatic patients with at least three atherothrombotic risk factors without statistically significant differences between men and women [134].
These five main randomized trials (CURE, CREDO, CLARITY-TIMI 28, COMMIT, CHARISMA) were included in a meta-analysis of focused on sex-related differences between men and women on dual antiplatelet therapy with aspirin versus clopidogrel versus aspirin alone [16]. Particularly in patients with known risk factors for CAD clopidogrel treatment was associated with a significant reduction in the risk of cardiovascular events (cardiovascular death, MI, or stroke). Nevertheless, merely the risk of MI was significantly reduced with clopidogrel (relative risk: 0.81; 95 % CI: 0.70–0.93) but not the risk for stroke or all-cause mortality, among the 23,533 women evaluated. On the contrary, significant reductions were shown in all 3 endpoints among the 56,091 men evaluated. Overall, the study found a significant 16 % relative reduction among men (7.8 % vs. 9.0 %, OR 0.84; 95 % CI 0.78–0.91) in major cardiovascular events with clopidogrel vs. placebo compared with a nonsignificant 7 % of relative reduction among women (11.0 % vs. 11.8 % OR 0.93; 95 % CI 0.86–1.01). No evidence of statistical heterogeneity between women and men was shown regarding to mortality, MI, stroke and major bleeding. However, much of the difference between men and women could be chance finding, and only a trend towards statistical heterogeneity based on gender was found (p = 0.092).
Variable responses to clopidogrel have been independently associated with polymorphisms of the cytochrome P450 (CYP) 2C19, in particular clopidogrel prodrug metabolism to its active metabolite. According with the presence of a gain-of-function and loss-of-function genotype, higher risks of both major bleeding [135] and stent thrombosis [136] have been detected respectively. There are not expected genotype-driven variances in outcome between men and women given that these polymorphisms are similarly distributed among men and women. Further investigations are still needed because it would be important to clarify if other gene-gene or gene-environment interactions could drive possible sex differences.
No significant interaction between sex and treatment assignment was found in the pivotal randomized trials of the novel antiplatelet agents prasugrel [17] and ticagrelor [18], despite the reductions in the primary endpoint relatively smaller in women than in men, these studies were not powered to examine treatment interactions among subgroups. Prasugrel is a third generation oral thienopyridine with more potent platelet P2Y12 inhibitory effects, because of the fact that a more favorable metabolic conversion produces higher concentrations of its active metabolite compared to clopidogrel [137]. In moderate to high-risk ACS patients undergoing PCI prasugrel was associated with a significant 19 % reduction in ischemic events compared to clopidogrel, in the Trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel– Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38) trial [17]. In the overall population, in spite of an increased risk of bleeding compared with clopidogrel, the net clinical benefit (defined as death from any cause, non-fatal MI, non-fatal stroke, and TIMI major hemorrhages) was favorable to prasugrel. Even with a higher absolute (2.4 % vs. 1.6 %) and relative (21 % vs. 12 %) reduction of the primary ischemic endpoint with prasugrel among men compared to women, no significant interactions between treatment and sex were found. On the other hand, underwriting the existence of less pharmacological variability compared with clopidogrel, a genetic subanalysis of the TRITON-TIMI 38 demonstrated that functional CYP genetic variants did not influence active metabolite levels, platelet inhibition and cardiovascular outcomes of 1,466 patients allocated to treatment with prasugrel [138]. Ticagrelor, the first member of a new class of reversible P2Y12 receptor antagonists called CycloPentylTriazoloPyrimidine (CPTP), demonstrated to be associated with a 16 % reduction in the composite ischemic endpoint compared to clopidogrel in patients with ST-elevation ACS who underwent primary PCI or with non-ST-elevation ACS planned for an invasive or medical approach in the phase III results of the Platelet Inhibition and Patient Outcomes (PLATO) trial [18]. Despite ticagrelor was associated with higher rates of major bleeding not related to coronary-artery bypass grafting, including more cases of fatal intracranial bleeding, in the overall population among ticagrelor and clopidogrel groups no differences in bleeding were found conferring to different definitions. Compared to clopidogrel, ticagrelor showed similar absolute (2.0 % vs. 1.9 %) and relative reduction (17.0 vs. 15.0 %) of the primary endpoint in women in comparison with men. Also among different sexes similar effects were also found regarding major bleeding.
Glycoprotein IIb/IIIa Inhibitors
GPI (abciximab, tirofiban and eptifibatide) block the binding of fibrinogen to the GP IIb/IIIa receptor on the surface of activated platelets and in this way inhibit the final common pathway leading to platelet aggregation. In vitro, no sex differences in platelet response to GPI were observed [19, 139]. Among the trials of GPI, a significant interaction between treatment and sex was found regarding cardiovascular events. In fact, between women and men, despite women had a higher rate of major and minor bleeding, no gender difference regarding major adverse outcomes was found at 30 days, 6 months and 1 year in a pooled analysis of data from three large randomized trials of patients undergoing PCI with adjunctive use of abciximab [20]. Also for eptifibatide the Enhanced suppression of the platelet GP IIb/IIIa receptor with Integrilin therapy (ESPRIT) trial, observed no statistical interaction between sex and treatment regarding death, MI or urgent target vessel revascularization either at 48 h or 1 year [21].
Six randomized trials on the efficacy and safety of GPI in patients with ACS not routinely scheduled for early coronary revascularization were included in a meta-analysis in which, despite a highly significant interaction between sex and use of GPI regarding cardiac events, GPI diminish the incidence of death or MI, particularly in patients at high risk of thrombotic complications [140]. Possibly because of the higher percentage of men with positive baseline troponins than women (49 % vs. 37 %), the interaction showed (an increased risk of 19 % in men vs. 15 % in women in the odds of 30-day death or MI compared with placebo or control), remained significant even after adjustment for baseline clinical characteristics, including age and co-morbidities (given that women were older, had more comorbid conditions, and more frequently had larger infarctions). Neither after risk stratification by troponin level sex differences were found. Actually, in both men and women with positive baseline troponins a reduction in the 30-day rate of death or MI by GPI was observed, while with negative troponins this effect was not beneficial in both sexes. Posteriorly in patients with UA/NSTEMI a different pattern of presenting biomarkers was observed [22]. Men were more likely to have elevated creatine kinase-MB and troponins, while women were more likely to have elevated CRP and brain natriuretic peptide. These findings suggested that a multimarker approach may be useful in the initial risk assessment of UA/NSTEMI, particularly in women. On the other hand, perhaps because of the concomitant use of clopidogrel, more recent investigations have not shown sex-related differences in outcome in ACS patients [141].
Anticoagulat Therapy
Anticoagulation in Coronary Artery Disease
The mentioned underrepresentation of women in clinical trials could be possibly related with an underestimation of cardiac risk or a misconception of symptoms of CAD and leads to the imperative necessity of gender-specific evidence related with the management and outcomes of ACS, particularly regarding differences in platelet reactivity and response to antithrombotic therapies [9, 54–59]. In fact, some investigations have already demonstrated many variances concerning women, such as different clinical presentation (symptoms and age), management or outcomes, different thrombotic profiles and response to antithrombotic therapies and propensity for increased bleeding [86–88]. In line with this, in addressing anticoagulant therapy in CAD, these need to be specifically addressed.
Indirect Thrombin Inhibitors
Unfractionated (UFH) and low-molecular-weight (LMWH) heparins enhance inactivation of factor Xa and, to a less extent, thrombin by binding to antithrombin III. The lack of necessity of laboratory monitoring of activity and the more predictable dose–response are the main advantages of LMWH. Another kinetic advantage of LMWH over UFH is that they inhibit the early steps of coagulation cascade and thrombin generation, due to a higher anti-factor Xa:IIa ratio and prolonged duration of anti-factor Xa activity. Sex is considered one of the clinical factors that affect the response to UFH, in addition to body weight, age, smoking history and diabetes mellitus [142, 143]. Particularly, in response to the anticoagulant effect of heparin women are expected to achieve higher activated partial thromboplastin time (aPTT) [23]. On the contrary, the pharmacokinetic (PK) and pharmacodynamic (PD) profiles after enoxaparin were found to be consistent between sexes in a post-hoc analysis of the Thrombolysis in Myocardial Infarction 11A (TIMI 11A) study, a multicenter dose-ranging trial to assess the safety of enoxaparin in patients with ACS [144, 145].
The Fragmin and Fast Revascularization during In- Stability in Coronary artery disease (FRISC) trial was the only placebo controlled trial of LMWH use reporting data stratified by sex [146]. This study compared dalteparin and placebo in patients with ACS, and it found that dalteparin was associated with a 63 % risk reduction in the composite of death and MI during the first 6 days, with a larger absolute (4.5 % vs. 2.2 %) and relative reduction (13.1 % vs. 28.9 %) of the primary endpoint in women compared with men. Nevertheless, regarding the frequency of minor bleedings, either with weight adjusted and fixed dose treatment it was higher in women compared with men. A significant interaction between sex and anti-Xa activity determined in samples obtained during the acute and the standard dosing phase of treatment was demonstrated in multiple regression analysis [25]. The FRISC II study reported a larger absolute and relative risk reduction in the composite endpoint of death and MI in women than in men [24].
A direct comparison between LMWH and UFH based on sex has been reported in few trials. In ACS patients the superiority of enoxaparin over UFH in reducing the risk of death and severe cardiac events in patients with ACS, was showed to be applicable to a plethora of subgroups in a pooled data meta-analysis of two large trials [26]. Nevertheless a significant benefit of enoxaparin over UFH regarding the double composite endpoint of death or MI was significantly observed in women, but not in man, despite a significant relative risk reduction was seen independently from sex with regards to the composite triple endpoint of death, MI or recurrent angina prompting urgent revascularisation [26]. Additionally, enoxaparin demonstrated to be not superior but also noninferior to UFH with a modest increase in the risk of major bleeding in the Superior Yield of the New Strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa Inhibitors (SYNERGY) study [147]. Multiple subgroups, including the ones stratified by sex and study site presented this absence of differences [147]. STEMI patients with programmed fibrinolysis where randomized to a regimen of enoxaparin or UFH as adjunctive antithrombin therapy in the Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment– Thrombolysis in Myocardial Infarction (ExTRACT-TIMI 25) study. In this study, women were older and more likely to have hypertension and diabetes. Despite increased rates of short term mortality, compared with men, women treated with enoxaparin had a similar relative benefit (16 % vs. 19 %) and a greater absolute benefit (2.9 % vs. 1.9 %) [27].
Direct Thrombin Inhibitors
Direct thrombin inhibitors (hirudin, bivalirudin, melagatran, argatroban, dabigatran, lepirudin and desirudin) block directly the interaction of thrombin with its substrates. Their property to decrease thrombin-mediated activation of platelets, consequently exerting some degree of antiplatelet effects, is one of the characteristics of this class of antithrombotic agents. In the guidelines for the management of non ST elevation ACS and for primary PCI in patients with STEMI, with or without pretreatment with heparin, bivalirudin is currently recommended as a class Ib [148, 149]. The safety and efficacy of bivalirudin in CAD have been observed in several registries and randomized trials supporting guidelines recommendations. The benefits associated with the administration of bivalirudin appear to be independent from sex. Therefore, it is likely that the higher risk of bleeding observed was related with baseline co-morbidities of women. In patients with stable or unstable angina undergoing PCI the Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events-2 (REPLACE-2) trial showed similar ischemic outcomes at 30 days for treatment with bivalirudin plus provisional GPI compared with heparin plus GPI, with similar mortality at 1 year, and a significant decrease of bleeding complications, without differences based on sex [150, 151]. Female gender demonstrated to be significantly associated with a 1.5-fold increased risk of major bleeding in a post-hoc multivariate analysis [30].
In patients with ACS undergoing an early invasive strategy also similar ischemic event rates at 30 days, less bleedings, and superior net clinical outcomes were associated with bivalirudin plus provisional GPI compared with heparin plus GPI in the aforementioned ACUITY trial. No significant interactions regarding net clinical outcomes, composite ischemia and major bleeding between the use of bivalirudin and numerous variables, including gender were observed in the subgroup analysis [152, 153]. Moreover, an almost twofold increased risk of major bleeding was demonstrated to be associated with female gender [31]. Among patients enrolled in the ACUITY, a further study specifically focused on sex-related difference trial corroborated these findings [100]. According the results of this study, women with ACS have higher rates of net clinical adverse event because of their higher risk of bleeding, but they do not experience an increased risk of one- and 12-month ischemic complications or mortality compared with men, even though differences in risk factors. Nevertheless, regardless of the treatment strategy chosen, bivalirudin monotherapy is related to similar short- and long-term protection from ischemic events and significantly less bleeding compared with a regimen of UFH plus GPI in women with ACS.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


