Methods of Administration and Warming
Intravenous fluids are administered via a sterile giving set to an IV cannula. Simple fluid-giving sets are suitable for slow to moderate flows of fluid and can be used with biometric pumps to ensure that the correct rate and volume of fluid is given. When faster flows are needed, wide bore sets are used. These often contain a screen filter (particle limit 170–200 µm) to prevent infusion of clumps of cells when infusing blood products.
Cold intravenous fluids cause hypothermia which impairs wound healing, blood clotting and increases metabolic demands, so fluids should be warmed whenever anything other than small volumes are being administered (NICE 2008). Fluid warmers work by heating IV fluid to body temperature by directly heating it or by running it by warm plates or warm water. Most systems have an additional chamber to collect air bubbles produced by the heated blood.
As IV fluid cannot currently carry oxygen, large blood losses must be replaced with transfused blood. As a guide, blood transfusion should be considered when 20% of the patient’s circulating volume has been lost, taking into account the patient’s haemoglobin level before and after the loss.
Blood volume is approximately:
- 70 mL/kg in adults (ideal body weight)
- 80 mL/kg in children
- 85–90 mL/kg in neonates (Cherian and Emmanuel 2002).
Special Considerations
Paediatrics
Fluid volumes need to be precise when dealing with children because of the small volumes involved. Children’s maintenance fluid in millilitres per hour is estimated by the formula: 4 mL/kg for the first 10 kg + 2 mL/kg for the next 10 kg + 1 mL/kg for the remainder of their weight (e.g. a 15 kg 3-year-old would receive (4 × 10) + (2 × 5) mL/h = 50 mL/h.
This fluid has a small volume so must be given precisely by a syringe pump, a volumetric pump or by using a volume-controlled burette which is filled with only the amount intended to be given.
Neurosurgery
The blood supply to the brain depends on the brain itself being at a lower pressure than the blood pressure. If the brain has swollen, the skull cannot expand so the pressure in the brain goes up. Mannitol (a 6-carbon sugar) is used as an osmotic diuretic to cause the pressure in the brain to decrease. Some units use hypertonic saline for the same purpose.
Dextrose-only solutions are not used for patients with head injuries or having neurosurgery because they increase cerebral oedema.
Blood Transfusion
Whole blood consists of red blood cells which carry oxygen, white blood cells which fight infection, platelets which are a key component the blood clotting system and plasma which contains further clotting factors and albumin. Donated whole blood is split into red cells, plasma and platelets. Red cells can be stored for 35 days. Plasma is frozen (fresh frozen plasma, ‘FFP’) or further processed to make cryoprecipitate which contains clotting factors and fibrinogen. Platelets can only be kept for 5 days.
Donated blood is screened for some viruses but still presents a risk to the recipient as the donor may have been in the incubation phase of a disease, or may have a disease which is not tested for. For this reason, and because of the expense, lack of availability and other complications such as transfusion related lung injury, great effort is made to avoid blood transfusion.
Blood groups and crossmatching
There are four ABO blood groups. These are genetically determined by the antigens in the blood (Table 11.2).
A patient cannot receive donated blood with antigens they do not possess or they will have a transfusion reaction which can lead to hypotension, renal failure and death (Table 11.3).
Table 11.2 The ABO blood groups with their red cell antigens and antibodies in plasma.
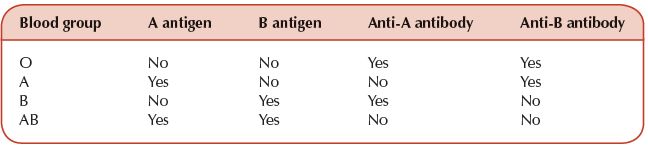
Table 11.3 Compatibility of ABO blood donors and recipients.

Group O blood can be given to anyone (O is the ‘universal donor’). An AB patient can receive any donated blood (AB is the ‘universal recipient’).
There are over 50 further antigens in the blood which can cause a transfusion reaction. If donated blood contains an antigen absent in the patient’s blood, the patient makes an antibody to it. The next time the patient receives blood containing this antigen, these antibodies attack the blood and cause a transfusion reaction.
Rhesus D
Ninety per cent of people have the Rhesus D antigen (Rh D positive). A Rhesus negative woman may carry a Rhesus positive baby. If there is mixing of fetal and maternal circulation (e.g. during delivery), the mother will produce antibodies which will affect any subsequent babies who are Rhesus D positive and give them severe anaemia. Women of childbearing age of unknown blood group should always receive Rhesus D negative blood in an emergency, and Rhesus D negative women receive injections of Anti D antibody to destroy any Rhesus D positive cells which may have leaked into their circulation during delivery or trauma (RCOG 2011).
When donated blood is selected for a recipient, the laboratory staff match the groups and antigens as closely as possible by testing the sample to see if it reacts with donated units of blood. If the laboratory has a recent blood sample demonstrating the patient has no antibodies then an electronic crossmatch may be possible which only takes a few minutes. Otherwise, a full crossmatch takes around an hour. If the situation is urgent, the laboratory can abbreviate the crossmatch to provide group-specific blood in about 15 minutes. If the situation is immediately life-threatening, the patient can be given emergency O negative (‘universal donor’) blood which is normally kept in the fridge in the emergency department, the operating theatres and the labour ward.
Other blood products
After donation, whole blood is split into plasma (which is often frozen), platelets and packed red cells. Packed red cells are usually suspended in a solution of saline, adenine, glucose and mannitol (SAGM) to keep them alive. As a rough guide, one unit of packed cells increases a patient’s haemoglobin by 1 g/dL. It has no clotting factors, so when more than two to four units are used, the patient will need to receive FFP and possibly platelets. Recent battlefield surgery experience indicates that using packed cells, FFP and platelets in a ratio of 1 : 1 : 1 may decrease mortality in major trauma but further evidence is needed before extrapolating this to civilian practice (Borgman et al. 2007).
Full crossmatching is not necessary for FFP transfusion but it should be group compatible. Donor plasma contains antibodies to any antigen not present in the person’s blood group (Table 11.2). Therefore, for FFP group AB (no antibodies) is the universal donor and O (no antigens for the antibodies to react to) is the universal recipient (Table 11.4).
Table 11.4 ABO compatibility of fresh frozen plasma donors and recipients.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


