Indications for Permanent and Temporary Cardiac Pacing
Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Introduction
Defects of cardiac impulse generation and conduction can occur at various levels in the cardiac conduction system. In general, intrinsic disease of the conduction system is often diffuse. For example, normal atrioventricular (AV) conduction cannot necessarily be assumed when a pacemaker is implanted for a disorder seemingly localized to the sinus node. Similarly, normal sinus node function cannot be assumed when a pacemaker is implanted in a patient with AV block. Conduction disorders that lead to important bradycardia or asytole may result from reversible or irreversible causes. Recognition of reversible causes is critical to avoid unnecessary commitment to long-term pacemaker therapy. This chapter reviews the common disorders that warrant cardiac pacing and lists the recommended indications set out by published guidelines.
Anatomy and Physiology of the Conduction System
For a complete understanding of rhythm generation, intracardiac conduction, and their pathology, a brief review of the anatomy and physiology of the specialized conduction system is warranted.
Sinus Node
The sinus node or sinoatrial (SA) node is a crescent shaped sub-epicardial structure located at the junction of the right atrium and superior vena cava along the terminal crest. It measures 10–20 mm (with larger extension in some studies) and has abundant autonomic innervation and blood supply, with the sinus node artery commonly coursing through the body of the node. Endocardially, the crista terminalis overlies the nodal tissue, although the inferior aspect of the node has a more sub-endocardial course. Histologically, the sinus node is comprised of specialized nodal cells (P cells) packed within a dense matrix of connective tissue. At the periphery, these nodal cells intermingle with transitional cells and the atrial working myocardium, with radiations extending toward the superior vena cava, the crista terminalis, and the intercaval regions.1,2 The absence of a distinct border and the presence of distal fragmentation explain the lack of a single breakthrough of the sinus node excitatory wavefront. The radiations of the node, although histologically distinct, are not insulated from the atrial myocardium. Hence, a clear anatomical SA junction is absent. The sinus node is protected from the hyperpolarizing effect of the surrounding atria, probably by its unique structure wherein electrical coupling between cells and expression of ion channels vary from the center of the node to the periphery. The pacemaker cells at the center of the node are more loosely coupled, while those at the periphery are more tightly coupled with higher density If (funny current; a slow sodium current) and INa currents.2
The SA node has the highest rate of spontaneous depolarization (automaticity) in the specialized conduction system and is responsible for the generation of the cardiac impulse under normal circumstances, although normal human pacemaker activity may be widely distributed in the atrium. The unique location of the sinus node astride the large SA nodal artery provides an ideal milieu for continuous monitoring and instantaneous adjustment of heart rate to meet the body’s changing metabolic needs. Impulse generation in the sinus node has been extensively studied in mammalian hearts, but remains incompletely understood. Sinus nodal cells have a low resting membrane potential of −50 to −60 mV. Spontaneous diastolic (phase 4) depolarizations are probably triggered by several currents, including If. T type calcium current is activated early during the diastolic depolarization, followed by the L type calcium current that triggers a “slow” action potential. Differential sensitivity to adrenergic and vagal inputs exists along the nodal pacemaker cells, such that superior sites tend to dominate during adrenergic drive while the inferior sites predominate during vagal stimulation.3 Interventions including premature stimulation, autonomic stimulation, and drugs have been shown to induce pacemaker shifts (due to multicentric origins) with variable exit locations.4
Atrioventricular Node
The compact AV node is a sub-endocardial structure situated within the triangle of Koch and measuring 5–7 mm in length and 2–5 mm in width.5,6 On the atrial side, the node is an integral part of the atrial musculature, in contrast to the AV bundle which is insulated within the central fibrous body and merges with the His bundle. Based on action potential morphology in rabbit hearts, atrial (A), nodal (N), and His (H) cells have been defined. Intermediate cell types such as AN and NH define areas toward the atrial and His bundle ends of the compact node, respectively. Histologically, the mid nodal part has densely packed cells in a basket-like structure interposed between the His bundle and the loose atrial approaches to the node. The AN cells are comprised primarily of transitional cells. Distinct electrical and morphological specialization is seen only in the progressively distal His fibers. Rightward and leftward posterior extensions of the AV node were described by Inoue and Becker.7 These extensions have clinical implications for defining re-entrant circuits that act as a substrate of AV nodal re-entrant tachycardia.
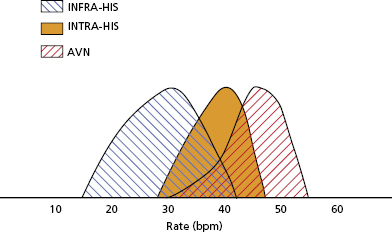
The AV node has extensive autonomic innervation and an abundant blood supply from the large AV nodal artery, a branch of the right coronary artery, in 90% of patients, and from the left circumflex artery in 10% (Figure 1.1). AV nodal conduction is mediated via “slow” calcium-mediated action potential and demonstrates decremental conduction due to post repolarization refractoriness as a result of delayed recovery of the slow inward currents. AV nodal tissue closer to the His bundle (NH and proximal His bundle area) generates junctional escape rhythms (Figure 1.2). Escape rates are dependent on the site of dominant pacemaker activity. Isoproterenol stimulation, for example, accelerates junctional escape and shifts the dominant activity to the transitional cells in the AN region and posterior extensions of the node.8–10
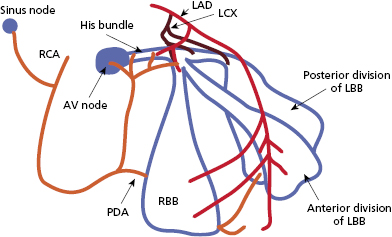
His–Purkinje System
Purkinje fibers emerging from the area of the distal AV node converge gradually to form the His bundle, a narrow tubular structure that runs through the membranous septum to the crest of the muscular septum, where it divides into the bundle branches. The His bundle has relatively sparse autonomic innervation, although its blood supply is quite ample, emanating from both the AV nodal artery and the septal branches of the left anterior descending artery (Figure 1.1). Longitudinal strands of Purkinje fibers, divided into separate parallel compartments by a collagenous skeleton, can be discerned by histological examination of the His bundle. Relatively sparse P cells can also be identified, embedded within the collagen. The rapid conduction of electrical impulses across the His–Purkinje system is responsible for the almost simultaneous activation of the right and left ventricles.
The bundle branch system is a complex network of interlaced Purkinje fibers that varies greatly among individuals. It generally starts as one or more large fiber bands that split and fan out across the ventricles until they finally terminate in a Purkinje network that interfaces with the myocardium (Figure 1.1). In some cases, the bundle branches clearly conform to a tri- or quadri-fascicular system. In other cases, however, detailed dissection of the conduction system has failed to delineate separate fascicles. The right bundle is usually a single, discrete structure that extends down the right side of the interventricular septum to the base of the anterior papillary muscle, where it divides into three or more branches. The left bundle more commonly originates as a very broad band of interlaced fibers that spread out over the left ventricle, sometimes in two or three distinct fiber tracts. There is relatively little autonomic innervation of the bundle branch system, but the blood supply is extensive, with most areas receiving branches from both the right and left coronary systems.
Indications for Permanent Pacemakers
Permanent pacing is considered in a number of clinical situations, some of which are unambiguous whereas others require a higher level of expertise for determination of potential benefit. However, two major factors determine the need for cardiac pacing: (1) symptoms associated with a bradyarrhythmia and (2) the site of conduction abnormality in the conduction system. In addition, the determination will depend on whether the conduction disease is likely to be permanent or reversible, such as due to a drug effect or acute inflammatory or ischemic process. A permanent pacemaker is generally a life-long commitment for a patient; the need for a generator changes and surgical revisions for malfunction become important considerations in younger patients. Hence, the decision to implant a pacemaker is not to be taken lightly.
A joint committee of the American College of Cardiology (ACC) and the American Heart Association (AHA) was formed in the 1980s to provide uniform criteria for pacemaker implantation. These guidelines were first published in 1984 and most recently revised in 2008 in conjunction with the Heart Rhythm Society (HRS).11 A task force for cardiac pacing and cardiac resynchronization therapy published similar guidelines in 2007.12 A recent focused update to the ACC/AHA/HRS guidelines of 2008 was published in 2012.13 It is recognized that there will be occasional cases that cannot be categorized based on these guidelines. Nevertheless, these guidelines have received wide endorsement and are periodically updated to incorporate important emerging data. A recent publication addressed the important issue of mode selection for cardiac pacing.14 This subject is discussed in detail in Chapter 3.
All guideline recommendations are subdivided into three classes to reflect the magnitude of treatment effect (Table 1.1). A class I indication pertains to a condition in which the procedure or intervention confers definite benefits. A class III indication is one where the intervention is not helpful and potentially harmful, and hence, not recommended.
| Class I | Conditions for which there is evidence and/or general agreement that a pacemaker implantation is beneficial, useful, and effective |
| Class II | Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of pacemaker implantation |
| Class IIa | Weight of evidence/opinion in favor of efficacy |
| Class IIb | Usefulness/efficacy less well established by evidence/opinion |
| Class III | Conditions for which there is evidence and/or general agreement that a pacemaker is not useful/effective and in some cases may be harmful |
Table 1.1 Classes of guideline recommendations
Additionally, the ACC/AHA Committee ranked evidence supporting its recommendations by the following criteria:
- Level A: Data derived from multiple randomized trials involving a large number of patients.
- Level B: Data derived from a limited number of trials involving a relatively small number of patients or from well-designed analyses of non-randomized studies or data registries.
- Level C: Recommendations derived from the consensus of experts.
Some class I indications will necessarily lack support from level A evidence due to early non-randomized studies documenting clear benefits such that randomized trials become unethical.
Sinus Node Dysfunction
Disorders of the sinus node can be divided into those primarily due to intrinsic pathology of the node and surrounding atrium, or extrinsic factors such as autonomic stimulation or drug effects. The terms sinus node disease (SND), sick sinus syndrome, and SA disease are often used interchangeably. All these refer to a broad range of abnormalities in the sinus node and atrial impulse formation and propagation (Table 1.2). They include persistent sinus bradycardia and/or chronotropic incompetence without identified cause, intermittent or persistent sinus arrest, and SA exit block. Frequently, atrial arrhythmias and sinus nodal dysfunction co-exist and cause symptomatic sinus pauses at cessation of an atrial arrhythmia (Figure 1.3). The term tachy–brady syndrome is applied because of the frequent need for bradycardia support with pacing to allow antiarrhythmic therapy for the tachycardia.
| Sinus bradycardia | Sinus rates persistently <60 bpm and associated with symptoms. Prolonged sinus node recovery time (following atrial pacing) is helpful in diagnosing sinus node disease but has low sensitivity |
| Chronotropic incompetence | Sinus rate does not increase with exertion. Diagnosis made with exercise test or ambulatory ECG monitoring |
| Sinoatrial (SA) block | Sinus beats are “dropped” in a regular pattern (e.g.2:1 SA block, 3:2 SA Wenckebach, etc.) due to blocking of impulses in the perinodal area between the sinus node and atrial muscle (by disease, medications, etc.). Diagnosis is made by the use of continuous ECG monitoring |
| Sinoatrial pause | Failure of impulse formation in the sinus node due to pathology, medications, etc. The diagnosis is made electrocardiographically by an absence of sinus P waves that occurs without any discernible pattern |
| Tachy–brady syndrome | The diagnosis is made electrocardiographically by alternating periods of sinus bradycardia and tachycardia (most commonly atrial fibrillation or flutter). The bradycardia is often manifested by periods of prolonged sinus arrest that often occur at termination of tachycardia |
Table 1.2 Manifestations of sinus node dysfunction and their diagnosis
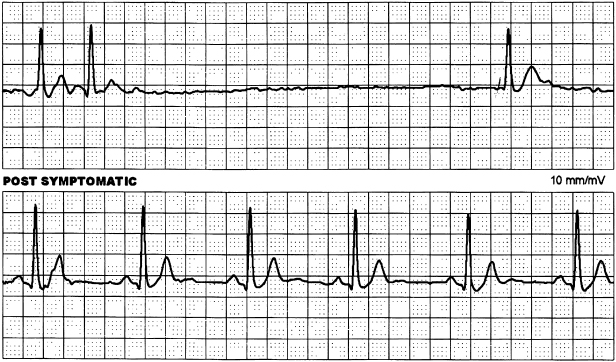
Pathology intrinsic to the sinus node is quite common, and its incidence increases with advancing age. Several patterns have been identified: A diffuse or localized atriopathy has been suggested. Electrophysiological studies have shown structural remodeling, particularly along the long axis of the crista terminalis, and associated with a more caudal migration of the atrial pacemaker activity.8 Progressive down-regulation of the ICaL channel and loss of connexin-43 expression are features in the guinea pig model.15 In humans, such atriopathy is also associated with atrial arrhythmias, particularly atrial fibrillation that develops in 50% of patients with SND. Atrial arrhythmias further aggravate SND and catheter ablation of fibrillation, and flutter has been shown to reverse some of the adverse electrical remodeling of the sinus node.16 Atrophic or hypoplastic sinus node has been described in association with congenital anomalies. A familial form of SND is also recognized. Finally, idiopathic SND without any detectable morphological abnormality can occur and may be related to abnormal neural innervation.
In patients with sinus node dysfunction, the correlation of symptoms with bradyarrhythmias is critically important. This is because there is a great deal of disagreement about the absolute heart rate or length of pause required before pacing is indicated. If the symptoms of SND are dramatic (e.g. syncope, recurrent dizzy spells, seizures, or severe heart failure), then the diagnosis may be relatively easy. However, symptoms are often non-specific (e.g. easy fatigability, depression, listlessness, early signs of dementia) and may be easily misinterpreted in the elderly. Electrophysiological studies have a low sensitivity for detection of SND and ambulatory monitoring with symptom correlation has the best diagnostic yield.
Essential drugs used for co-existing conditions can accentuate SND (Figure 1.4). If cessation of a drug is anticipated to cause deterioration of the primary condition, permanent pacing may be needed to allow continuation of medical therapy in some patients. A group of patients has been identified who have a relatively fixed heart rate during exercise; this condition is referred to as chronotropic incompetence and can result in marked exercise intolerance in some patients. Heart rate response to exercise compared with that of an age- and gender-matched population is often necessary for clear diagnosis, although no specific parameter has been established as a diagnostic standard.
Patients with SND may have associated disease in the AV node and His–Purkinje conduction system. However, the rate of lone SND progressing to AV block is low. The mean annual incidence of complete AV block developing in patients implanted with AAI pacemakers for SND is 0.6% (range 0–4.5%) with an overall prevalence of 2.1% (range 0–11.9%).11 The natural history of untreated SND is highly variable. Syncope resulting from sinus arrest tends to be recurrent and may result in falls and significant orthopedic injuries, especially in the elderly. The incidence of sudden death is low and SND very rarely affects survival regardless of whether or not it is treated with a pacemaker.
Indications for Permanent Pacing in Sinus Node Dysfunction
Class I Indications
- Sinus node dysfunction with documented symptomatic bradycardia or sinus pauses. (Level of evidence: C)
- Symptomatic chronotropic incompetence. (Level of evidence: C)
- Sinus node dysfunction as a result of essential long-term drug therapy of a type and dose for which there are no acceptable alternatives. (Level of evidence: C)
Class IIa Indications
- Sinus bradycardia with a heart rate of less than 40 bpm when a clear symptom correlation has not been established with documented bradycardia. (Level of evidence: C)
- Syncope of unexplained origin when clinically significant abnormalities of sinus node function are detected or provoked during electrophysiological studies. (Level of evidence: C)
Class IIb Indications
- In minimally symptomatic patients with persistent bradycardia with a heart rate of less than 40 bpm during awake hours. (Level of evidence: C)
Class III (Permanent Pacing Not Indicated)
- Permanent pacing is not indicated in asymptomatic patients with SND. (Level of evidence: C)
- Sinus node dysfunction in patients with symptoms suggestive of bradycardia that are clearly documented as not associated with a slow heart rate.
- Sinus node dysfunction with symptomatic bradycardia due to non-essential drug therapy.
Acquired Atrioventricular Block
In the majority, sclerodegenerative changes account for progressive conduction system disease. However, in a significant proportion, AV block is secondary to other causes that are potentially reversible or associated with progressive heart disease with added risk of ventricular arrhythmias such that an implantable cardioverter–defibrillator (ICD) should be considered as a means of providing pacing therapy. In a recent review of unexplained heart block in patients under 55 years of age, cardiac sarcoidosis or giant cell myocarditis accounted for 25% of cases and these patients had a high incidence of sudden death, ventricular tachycardia, or need for cardiac transplantation.17 In younger patients presenting with advanced conduction system disease, further evaluation with cardiac magnetic resonance (CMR) imaging or positron emission tomography (PET) is useful for detection of pathology that merits the use of an ICD as opposed to provision of cardiac pacing alone.
Based on electrocardiography (ECG) characteristics, AV block is classified as first, second, and third degree. Anatomically, block can occur at various levels in the AV conduction system; above the His bundle (supra-His), within the His bundle (intra-His), and below the bundle of His (infra-His). First-degree AV block is defined as abnormal prolongation of the PR interval to greater than 200 ms and is commonly due to delay in the AV node irrespective of QRS width. Type I second-degree heart block refers to progressive PR prolongation before a non-conducted beat and a shorter PR interval after the first blocked beat. This is the classical Wenckebach type AV block usually seen in conjunction with narrow QRS complexes, implying a more proximal level of block, usually in the AV node (Figure 1.5). Type II second-degree heart block is characterized by fixed PR intervals before and after blocked beats, and is usually associated with a wider QRS complex, indicating distal levels of block in the conduction system. Type II second-degree AV block is usually below the level of the AV node (within or below the His bundle); symptoms and progression to complete AV block are common. AV conduction in a 2:1 pattern can be due to proximal or distal block, although the width of the QRS can help differentiate these based on the above principle. Advanced second-degree block or “high-grade” AV block refers to two or more non-conducted sinus P waves, but with resumption of conducted beats suggesting preservation of some AV conduction (Figure 1.6). In the setting of atrial fibrillation or flutter, a prolonged pause (e.g. >5 s) is often due to advanced second-degree AV block. Third-degree AV block is defined as the absence of AV conduction. In the case of atrial fibrillation, complete AV block often manifests as a regularized slow ventricular rate.
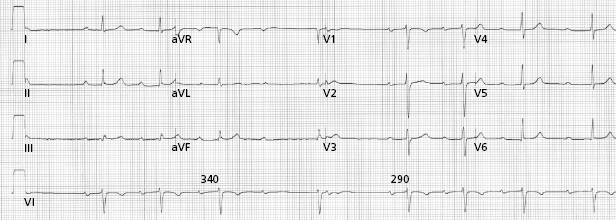
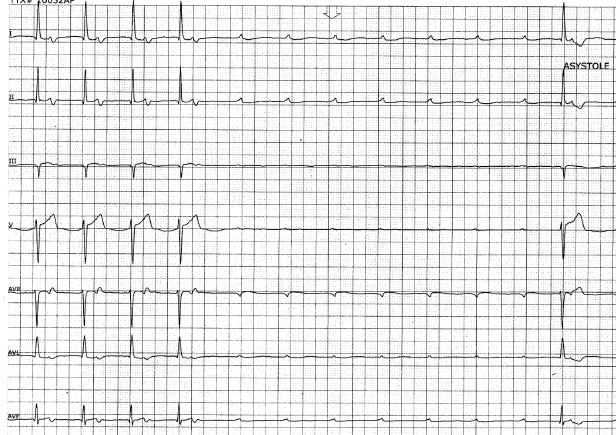
The site of AV block will to a great extent determine the adequacy and reliability of the underlying escape rhythm (Figure 1.7 and Figure 1.8). While ECG characteristics are helpful in defining levels of block, they are not always reliable and occasionally, an electrophysiological study is required. Type I second-degree block, for example, can occasionally be infranodal, even with a narrow QRS, and may warrant the consideration of pacing.18 Certain clinical maneuvers may be helpful in determining the level of block. Increased AV conduction with exercise and atropine generally indicate block at the AV nodal level, while maneuvers that slow the atrial rate, such as carotid massage, improve His–Purkinje conduction by allowing for recovery from refractoriness (Table 1.3).
| Block above AV node | Block below AV node | |
|---|---|---|
| Exercise | + | ± or − |
| Atropine | + | ± or − |
| Carotid sinus massage | − | + or ± |
| Isoproterenol | + | − or ± |
+, improved AV conduction; −, worsened AV conduction.
Table 1.3 Responses to maneuvers to identify level of block in patients with 2:1 atrioventricular (AV) block
There is considerable variation in the symptomatic manifestation of AV block, ranging from an asymptomatic status to syncope and sudden death. First-degree AV block and asymptomatic type I second-degree AV block are in general benign and not an indication for cardiac pacing. However, rarely, first-degree block with marked PR prolongation can potentially cause atrial systole to occur in close proximity to the preceding ventricular systole and give rise to symptoms similar to those of a pacemaker syndrome.19 Prolongation of the PR interval is particularly important in patients with left ventricular (LV) dysfunction as marked PR prolongation in excess of 250–300 ms can lead to impaired LV filling, increased pulmonary capillary wedge pressure, and decreased cardiac output.20 Similar consequences can ensue in patients with type I second-degree AV block even in the absence of bradycardia-related symptoms.
Type II second-degree AV block is important as it has a high rate of progression to third-degree AV block. It usually reflects diffuse conduction system disease and often warrants permanent pacing even in the absence of symptoms. Third-degree AV block with a wide QRS escape rhythm, often present with fatigue, dyspnea, pre-syncope or frank unheralded syncope. Rarely, ventricular fibrillation and torsades de pointes ventricular tachycardia (VT) can result from marked bradycardia and prolonged pauses. Permanent cardiac pacing should be strongly considered even if the escape rate is greater than 40 bpm, because it is not necessarily the escape rate that determines a safe and reliable heart rhythm but the site of origin of the escape rhythm. Infra-His escape rhythms are more likely associated with prolonged asystole, syncope, and death (Figure 1.8).
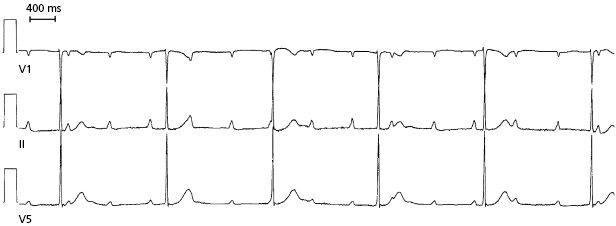
AV block, usually with 2:1 AV conduction, can be provoked by exercise (Figure 1.9). Patients typically complain of exertional dyspnea and dizziness. The abnormality is often reproducible by exercise testing. Once ischemia is excluded as a cause, permanent pacing is remarkably effective for symptom relief. Without pacing, these patients have a poor prognosis because the site of conduction block is below the AV nodal level.21
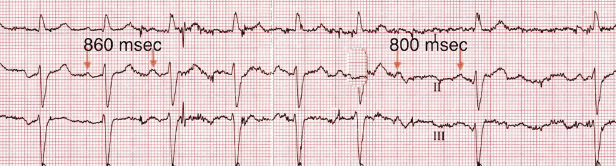
A distinct form of paroxysmal AV block associated with syncope has been described in patients without structural heart disease or evidence for conduction disturbance on ECG.22 Patients present with abrupt syncope associated with abrupt onset of high-grade AV block and prolonged asystole, with recovery of normal AV conduction soon afterward. Electrophysiological studies do not indicate the presence of His–Purkinje disease. The majority tends to have an exaggerated response to intravenous adenosine, raising the possibility of a variant of reflex syncope (see “Reflex syncope”). However, the classical sinus slowing prior to onset of AV block that is typical of vagally-mediated AV block, is usually absent in this group of patients.
In general, the presence of symptoms documented to be due to AV block is an indication for permanent pacing regardless of the site of the block (e.g. above the His bundle as well as below the His bundle). However, it is important to recognize potentially reversible causes of AV block despite their presentation with symptoms. Important examples include acute myocarditis (particularly that associated with Lyme carditis), AV block related to drug toxicity, transient vagotonia, and hypoxic events. Many of these conditions tend to resolve with disease-specific treatment and although temporary pacing may be required, permanent pacing is seldom necessary. One exception is drug-related AV block that may not always resolve completely on cessation of the medication and may need permanent pacing (see “Temporary pacing indications”). The indications for permanent pacing of heart block due to acute myocardial infarction (MI), congenital AV block, and increased vagal tone differ and are discussed in “Reflex syncope,” “Congenital complete AV block,” and “Permanent pacing after acute myocardial infarction.”
Indications for Permanent Pacing in Acquired AV Block
Class I Indications
- Third-degree and advanced second-degree AV block at any anatomical level, associated with any one of the following conditions:
- Bradycardia with symptoms (including heart failure) presumed to be due to AV block. (Level of evidence: C)
- Arrhythmias and other medical conditions requiring drugs that result in symptomatic bradycardia. (Level of evidence: C)
- Documented periods of asystole of 3.0 s or longer, any escape rate of less than 40 bpm or with any escape rhythm below the AV node in awake, symptom-free patients. (Level of evidence: C)
- Atrial fibrillation and bradycardia with one or more pauses of at least 5 s or longer in awake, symptom-free patients. (Level of evidence: C)
- Following catheter ablation of the AV junction. (Level of evidence: C)
- Postoperative AV block that is not expected to resolve after cardiac surgery. (Level of evidence: C)
- Neuromuscular diseases with AV block, such as myotonic muscular dystrophy, Kearns–Sayre syndrome, Erb muscular dystrophy, and peroneal muscular atrophy, with or without symptoms, because there may be unpredictable progression of AV conduction disease. (Level of evidence: B)
- Bradycardia with symptoms (including heart failure) presumed to be due to AV block. (Level of evidence: C)
- Second-degree AV block regardless of type or site of block, with associated symptomatic bradycardia. (Level of evidence: B)
- Third-degree AV block with evidence for cardiomegaly or LV dysfunction. (Level of evidence: B)
Class IIa Indications
- Persistent third-degree AV block with an escape rate of greater than 40 bpm in asymptomatic adult patients without cardiomegaly. (Levels of evidence: C)
- Asymptomatic type II second-degree AV block at intra-His or infra-His levels found at electrophysiological study. (Level of evidence: B)
- First- or second-degree AV block with symptoms similar to those of pacemaker syndrome or hemodynamic compromise. (Level of evidence: B)
- Asymptomatic type II second-degree AV block with narrow QRS. Note that when type II second-degree AV block occurs with wide QRS, including isolated right bundle branch block (RBBB), pacing becomes a class I indication. (Level of evidence: B)
Class IIb Indications
- Neuromuscular diseases such as myotonic muscular dystrophy, Kearns–Sayre syndrome, Erb muscular dystrophy, and peroneal muscular atrophy with any degree of AV block (including first-degree AV block), with or without symptoms, because there may be unpredictable progression of AV conduction disease. (Level of evidence: B)
- AV block in the setting of drug use and/or toxicity when the block is expected to recur even after the drug is withdrawn. (Level of evidence: B)
Class III (Not Indicated)
- Asymptomatic first-degree AV block. (Level of evidence: B)
- Asymptomatic type I second-degree AV block at the AV nodal level or not known to be intra- or infra-Hisian. (Levels of evidence: C)
- AV block expected to resolve and/or unlikely to recur (e.g. drug toxicity, Lyme disease, or transient increases in vagal tone or during hypoxia in sleep apnea syndrome in the absence of symptoms). (Level of evidence: B)
Chronic Bifascicular Block
In bifascicular block, the ECG shows evidence of conduction delay in both bundles such as complete RBBB with anterior or posterior hemiblock or complete left bundle branch block (LBBB) alone. The term alternating bundle branch block (BBB; or bilateral BBB) refers to evidence for impaired conduction in the right bundle and both fascicles of the left bundle on successive ECGs. In strict terms, evidence for disease in all three fascicles should justify the term trifascicular block. However, the term trifascicular block has also been loosely applied to bifascicular block with first-degree AV block where the block may actually be due to either or a combination of AV nodal and infra-His conduction disease.
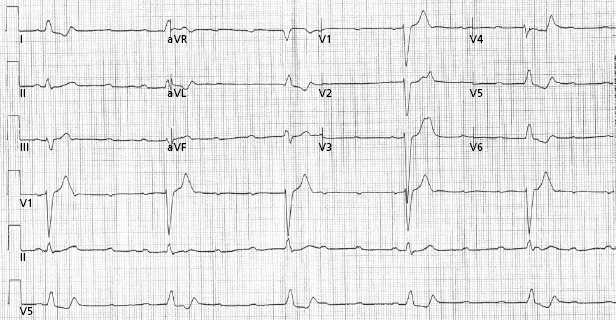
The prevalence of BBB increases with age (approximately 1% in middle age and rising to 17% at age 80).23 LBBB is less common but its presence is associated with a higher incidence of structural heart disease. In bifascicular block, the risk of progression to advanced heart block is related to the presence of symptoms. Syncope is the sole predictor. In the absence of syncope, the annual incidence is 0.6–0.8%, whereas syncopal patients have a 5–11% annual risk of progression to AV block.24,25 The finding of an His–ventricular (HV) interval of greater than 100 ms or the demonstration of intra- or infra-Hisian block during incremental atrial pacing at a rate of less than 150 bpm is highly predictive for the development of high-grade AV block (Figure 1.10), but their prevalence is low and hence, sensitivity is low.26,27 Care has to be exercised during atrial pacing so as not to misinterpret physiological AV block that is often seen with long–short intervals. The majority of patients with bifascicular block who undergo electrophysiological studies will have normal or mildly prolonged HV intervals. However, in patients with BBB and normal electrophysiological study, implantable loop monitors have shown that recurrent syncope is often due to a bradyarrhythmia, most commonly sudden onset paroxysmal AV block.28 Hence, unexplained syncope is a better indicator of the need for pacing than electrophysiological studies.
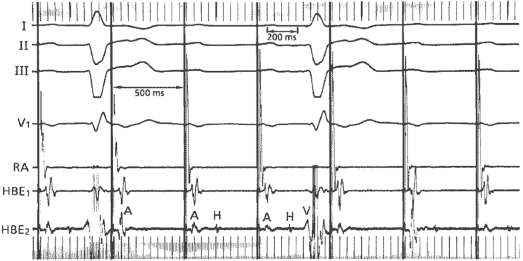
Because chronic bifascicular block is associated with other forms of heart disease, pacing alone, although successful for symptom relief, has not been shown to improve mortality. In the presence of ventricular dysfunction, ventricular tachycardia is an alternative mechanism for syncope and sudden death. Programmed stimulation of the ventricle may demonstrate inducibility for ventricular arrhythmia, necessitating the consideration of an ICD.
Indications for Pacing in Chronic Bifascicular Block
Class I Indications
- Advanced second-degree AV block or intermittent third-degree AV block. (Level of evidence: B)
- Type II second-degree AV block. (Level of evidence: B)
- Alternating BBB. (Level of evidence: C)
Class IIa Indications
- Syncope not demonstrated to be due to AV block and when other likely causes have been excluded, specifically ventricular tachycardia. (Level of evidence: B)
- Incidental finding at electrophysiology study of markedly prolonged HV interval (≥100 ms) in asymptomatic patients. (Level of evidence: B)
- Incidental finding at electrophysiology study of pacing-induced infra-His block that is not physiological. (Level of evidence: B)
Class IIb Indications
- Neuromuscular diseases such as myotonic muscular dystrophy, Kearns–Sayre syndrome, Erb muscular dystrophy, and peroneal muscular atrophy with any degree of fascicular block with or without symptoms, because there may be unpredictable progression of AV conduction disease. (Level of evidence: C) (Note that this is a class IIa indication in European guidelines.)
Class III (Not Indicated)
- Fascicular block without AV block or symptoms. (Level of evidence: B)
- Fascicular block with first-degree AV block without symptoms. (Level of evidence: B)
Reflex Syncope
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


