7 Parenchymal Lung Disease OBJECTIVES
 Understand the physiologic derangements that lead to diffuse parenchymal lung diseases.
Understand the physiologic derangements that lead to diffuse parenchymal lung diseases.
 Integrate the pathophysiology of diffuse parenchymal lung diseases to understand clinical disease presentations.
Integrate the pathophysiology of diffuse parenchymal lung diseases to understand clinical disease presentations.
 Understand the therapy and prognosis of diffuse parenchymal lung disease.
Understand the therapy and prognosis of diffuse parenchymal lung disease.
GENERAL CONSIDERATIONS
Definition
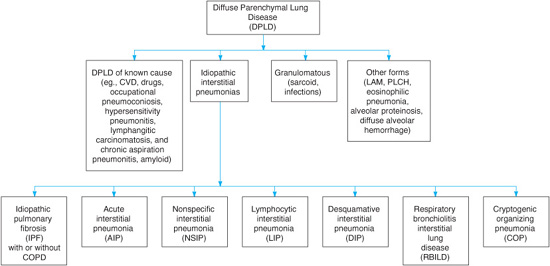
 Diffuse parenchymal lung diseases (DPLDs) are a heterogeneous group of nonneoplastic disorders resulting from damage to the lung parenchyma by varying patterns of inflammation and fibrosis. These disorders are also known as interstitial lung diseases, but these injuries not only involve the interstitium (the space between the epithelial and endothelial basement membranes), but also the air spaces, peripheral airways, and blood vessels, and therefore DPLD is a more appropriate term. One of the problems of dealing with DPLDs is that the terminology for different histopathologic entities and clinical syndromes has become confusing and is littered with acronyms. For idiopathic fibrosis alone there are more than 20 synonyms. An international multidisciplinary committee has standardized the classification of idiopathic interstitial pneumonias. The terminology put forth by the committee will be used here (Figure 7–1).
Diffuse parenchymal lung diseases (DPLDs) are a heterogeneous group of nonneoplastic disorders resulting from damage to the lung parenchyma by varying patterns of inflammation and fibrosis. These disorders are also known as interstitial lung diseases, but these injuries not only involve the interstitium (the space between the epithelial and endothelial basement membranes), but also the air spaces, peripheral airways, and blood vessels, and therefore DPLD is a more appropriate term. One of the problems of dealing with DPLDs is that the terminology for different histopathologic entities and clinical syndromes has become confusing and is littered with acronyms. For idiopathic fibrosis alone there are more than 20 synonyms. An international multidisciplinary committee has standardized the classification of idiopathic interstitial pneumonias. The terminology put forth by the committee will be used here (Figure 7–1).
Normal Structure & Function
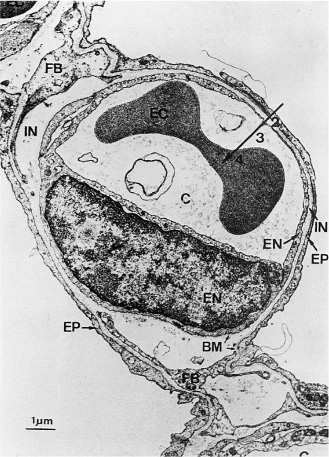
The basic structural unit of the lung is the alveolar-capillary membrane, which is the site of respiratory gas exchange. In the terminal air spaces of the lung, the pulmonary capillaries are closely apposed to the alveolar walls, giving the pulmonary circulation a large surface area where gas exchange can take place. The overall alveolar surface area is approximately 100–140 m2, an area at least 50-fold greater than the surface area of the skin. The alveolar-capillary membrane is normally a delicate structure composed of the alveolar epithelium (primarily type I alveolar squamous epithelial cells), the epithelial basement membrane, the interstitial stroma, the endothelial basement membrane, and the capillary endothelium. Type I alveolar epithelial cells constitute more than 90% of the alveolar surface area. Also present in the alveolar spaces are type II alveolar epithelial cells, which are the progenitor cells of type I cells during gestation and after several types of lung injuries. Type II pneumocytes reside at the corners of the alveolar septae, and due to their cuboidal shape they constitute only a small portion of the alveolar surface area (less than 10%), although they outnumber the type I cells by two-fold. They are the cells responsible for production and storage of surfactant, which forms a thin layer on the alveolar surface. Thus, for gas exchange to occur, oxygen must diffuse from the alveolar space through the surfactant layer, the epithelial cell, the epithelial basement membrane, the interstitial tissue, the endothelial basement membrane, the endothelial cell, the plasma, and the red blood cell membrane and cytoplasm to reach the molecule of hemoglobin that will bind it for transport. Carbon dioxide follows the pathway in the opposite direction to reach the alveolar space, from which it is exhaled. In the normal lung, the alveolar-capillary unit (ie, a representative alveolar septum) is of the order of 8–12 μm in thickness which, given the rapid rates of diffusion of oxygen and carbon dioxide, does not pose a significant barrier to gas exchange (Figure 7–2). Also present in the alveolar spaces are the resident scavenger cells of the lung, the alveolar macrophages. These cells are mobile phagocytes that roam the alveolar surfaces and represent the initial line of defense against invading pathogens or toxins. If unable to clear the offenders, they are also quite active metabolically and are capable of initiating inflammatory responses to the offending agent.
Figure 7–1. Classification of diffuse parenchymal lung diseases (DPLDs). CVD, cardiovascular disease; LAM, lymphangioleiomyomatosis; PLCH, pulmonary Langerhans cell histiocytosis; COPD, chronic obstructive pulmonary disease. (Modified with permission from the American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2002;165:277–304.)
ETIOLOGY & PATHOGENESIS
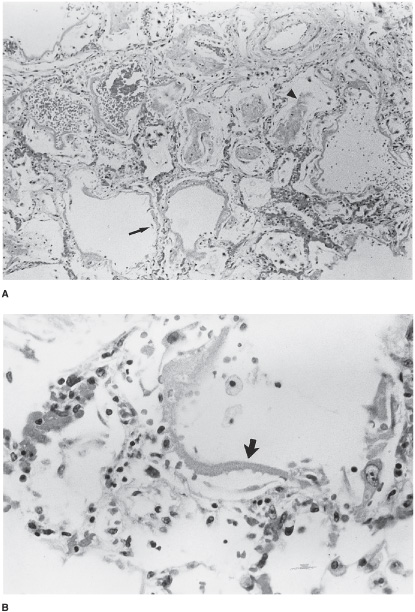
Several patterns of lung injury can occur. If the injury is related to a distinct exposure or clinical scenario, such as a severe toxic inhalational exposure or an episode of bacterial septic shock or severe pancreatitis, widespread alveolar damage may result from the inflammatory reaction, with cellular activation and release of inflammatory mediators. The histologic pattern is known as diffuse alveolar damage (DAD). The clinical correlate of this lung injury is the acute respiratory distress syndrome (ARDS), which is characterized by severe respiratory distress. There is extensive endothelial and epithelial destruction, with flooding of the alveolar spaces with inflammatory exudates. This pattern occurs acutely regardless of the cause of the damage. There is disruption of the epithelial layer of type I alveolar pneumocytes, resulting in exposed basement membrane, as well as defects in surfactant function and synthesis (Figure 7–3). With loss of surfactant function, increases in surface tension may result in localized areas of alveolar collapse or atelectasis; these areas may contribute to ventilation-perfusion mismatching and shunt or shunt-like states. Accompanying the destruction of the alveolar-capillary barrier, there is exudation of inflammatory proteinaceous material into the air spaces, with fibrin deposition and the formation of hyaline membranes. Inflammatory cells, including polymorphonuclear leukocytes and mononu-clear cells, are recruited (in response to chemotactic agents released during the inflammatory process) into the alveoli within hours of the inciting injury. This process differs from acute bacterial pneumonia, which is characterized by influx of primarily polymorphonuclear leukocytes with a fibrinous exudate and possibly a component of hemorrhage. Necrosis may occur in severe cases. However, acute bacterial infections generally present with sufficiently distinct clinical signs and symptoms that they are not often confused with interstitial pneumonitis.
Figure 7–2. Transmission electron photomicrograph of the normal alveolar-capillary structure. Structures shown in this cross-section are the capillary (C) with an endothelial cell (EN, including nucleus), an alveolar epithelial cell (EP), the thin interstitial space (IN), the basement membrane (BM), and fibroblast processes (FB). As oxygen diffuses from the alveolus into the bloodstream, it traverses the alveolar-capillary barrier (2), the plasma (3), and the erythrocyte (4). (Reproduced with permission from Weibel E and Respiration Physiology, Vol. 11; Weibel ER. Morphometric Estimation of Diffusion Capacity, pp 54–75, 1970/71 with kind permission of Elsevier Science NL, Sara Burgerhartstraat 25, 1055 KV Amsterdam, the Netherlands.)
When acute lung injury results from a well-defined insult that occurs at a specific time, the histologic pattern of the alveolar injury is more homogeneous than would occur if the insult were ongoing or even of a chronic, recurring nature. In the first instance, a lung biopsy would reveal most of the injury to be in the same stage (initial acute exudative phase or a reparative phase), with relatively less heterogeneity than when the injury is of an ongoing nature. Ongoing injury may occur with the repeated administration of drugs or radiation, which are toxic and provoke the injury. In such a situation, the histopathologic picture is that of acute injury superimposed on various phases of the reparative process. Idiopathic pulmonary fibrosis (IPF) (in which the inciting agent cannot be identified) is characterized by ongoing injury in the setting of the repair process that results in fibrosis and scarring rather than restoration of normal architecture. Recent studies suggest that the fibrotic responses in the lung relate to dysregulated immune or inflammatory responses. Alveoli are lost in this process of ongoing inflammation and scar formation (Figure 7–4). Therefore, the histologic hallmark of IPF is usual interstitial pneumonia (UIP). UIP is characterized by temporal heterogeneity. The histology is a heterogeneous appearance with alternating areas of normal lung, interstitial inflammation, fibrosis, and honeycomb change. Scattered foci of proliferating fibroblasts (fibroblastic foci) are also present. These represent areas of active fibrosis.
Figure 7–3. Histopathology of diffuse alveolar damage (DAD). A. Low-power view reveals thickening of alveolar septae from edema and inflammatory infiltration (arrow), and alveolar space hemorrhage and fibrin membrane formation (arrowhead). B. Higher power view of hyaline membrane (arrow).
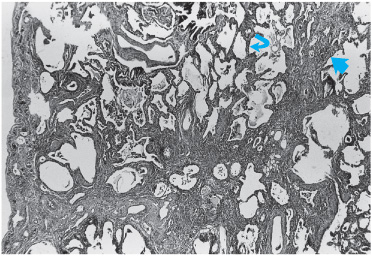
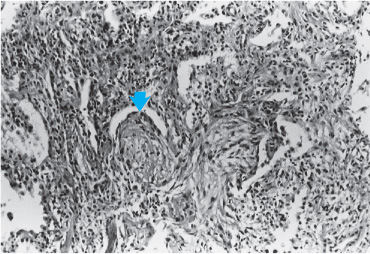
DAD and pulmonary fibrosis constitute one response of the lung to injury, but other patterns can occur. Many inciting agents have been described as resulting in a pattern of lung injury known as organizing pneumonia with or without organization within the bronchioles (polypoid bronchiolitis obliterans). This pattern was formerly known as bronchiolitis obliterans with organizing pneumonia. An idiopathic variety has been described as cryptogenic organizing pneumonia (COP). In this injury, the primary site of damage is the bronchiolar epithelium, and the injury results in the formation of connective tissue plugs within the terminal bronchioles, which can also extend distally to fill the alveolar ducts (Figure 7–5). Rather patchy in nature, an inflammatory exudate consisting of mononuclear cells in the peribronchiolar areas accompanies the fibrosis that occurs in bronchioles and alveolar ducts. Macrophages in the alveoli adjacent to involved areas may accumulate and take on a foamy appearance, simulating the appearance of lipoid pneumonia. An important feature of organizing pneumonia is that the lesions are all uniform in nature, suggesting that they are temporally homogenous. In order for the diagnosis of organizing pneumonia to be confirmed, it must be the predominant pattern on biopsy with the appropriate clinical presentation. Histologic changes of organizing pneumonia may be seen in the setting of other types of injury, including DAD and IPF. However, for the diagnosis of this distinct entity to be made, it must be the predominant histopathologic pattern.
Figure 7–4. Histopathology of idiopathic pulmonary fibrosis (IPF). Features are destruction of alveolar structures and thickened alveolar septae (curved arrow), with inflammatory cell infiltration and fibrosis (large arrow).
Another pattern of lung injury is bronchiolitis obliterans, also known as constrictive bronchiolitis. This pattern of injury only involves the bronchioles and is not a true DPLD. Most commonly recognized following organ transplantation (lung, heart-lung, and bone marrow transplantations), constrictive bronchiolitis can also occur as an idiopathic disorder, accompanying collagen vascular diseases, after infections, or as a result of drug or toxin exposure. Bronchiolitis obliterans has been associated with exposure to the artificial butter flavoring Diacetyl among workers in a microwave popcorn plant. Histopathologically, this injury is characterized by peribronchiolar mononuclear cell inflammatory infiltration and concentric fibrosis of the terminal bronchioles (Figure 7–6). If this process is progressive, it leads to narrowing and gradual obstruction at the bronchiolar level. Histopathologic changes are usually limited to the bronchioles, and there is little involvement of the distal parenchyma. Chronically, there may be accompanying bronchiolar dilatation with mucus stasis. Because of the tendency for this disorder to occur in conjunction with organ transplantation, hypotheses of the cause have been developed. Bronchiolar epithelial injury may underlie the process, either due to alloimmune-dependent and/or non-alloimmune mechanisms that result in injury and inflammation of the epithelial cell and subepithelial structures resulting in fibrosis of the small airways. However, the pathophysiology remains uncertain. If the injury is progressive and uninterrupted, excessive fibroproliferation due to ineffective epithelial regeneration and severe small airway obstruction may result, which is often refractory to current therapies.
Figure 7–5. Histopathology of proliferative bronchiolitis. Note extensive inflammatory infiltrate and connective tissue plugs filling the lumens of terminal bronchioles (arrow).
Figure 7–6. Histopathology of constrictive bronchiolitis. High-power view of terminal bronchiole concentrically narrowed with near obliteration of airway lumen (arrow). (Modified and reproduced with permission from Colby TV: Bronchiolar pathology. In: Epler GR, ed. Diseases of the Bronchioles. New York, Raven Press, 1994, pp 77–100.)
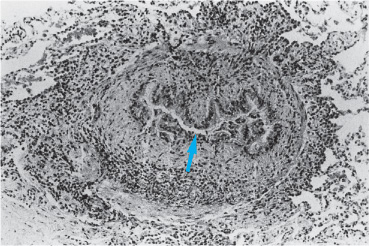
Another response of the lung to insult is the formation of granulomatous inflammation. While usually seen in the setting of certain infections (eg, tuberculosis or fungal infections), pneumonitis due to hypersensitivity to antigens may also result in the formation of granulomas. In addition, an idiopathic variety known as sarcoidosis may occur. Presumably a reaction to an antigen, recognized or not, this disease is accompanied by widespread granuloma formation (involving lymph nodes, spleen, liver, skin, and/or the eyes). The lung is frequently involved. Granulomatous inflammation characteristically is patchy in distribution and includes components of granulomas and mononuclear cell alveolitis as well as areas of fibrosis. A granuloma is a structure composed of epithelioid cells derived from monocytes and macrophages and is presumably formed by the cell-mediated reaction to an antigen. It has a small nodular center composed of mononuclear cells and fibroblasts with or without necrosis (necrosis is typically not a feature of sarcoidosis, but may accompany infectious causes) (Figure 7–7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree