6
Obstructive Lung Disease
OBJECTIVES
 Define airway obstruction and understand its pathophysiologic relationship to various disease processes.
Define airway obstruction and understand its pathophysiologic relationship to various disease processes.
 Review the clinical features of the various diseases that present with airway obstruction.
Review the clinical features of the various diseases that present with airway obstruction.
GENERAL CONSIDERATIONS
Airway obstruction can be defined as any abnormal reduction in airflow. Resistance to airflow can occur anywhere in the airway from the upper airway to the terminal bronchi and is characteristic of asthma and chronic bronchitis. Although diseases that cause airway obstruction have a common physiologic effect, their pathogenesis and pathophysiologic mechanisms may be quite different. Airflow limitation may also be the result of loss of elastic recoil due to tissue destruction, as seen in emphysema. Despite this distinction in pathogenesis and pathophysiology, the diseases are sometimes difficult to distinguish clinically. This chapter will focus on asthma, chronic obstructive pulmonary disease (COPD)/emphysema, and bronchiectasis with some reference to other causes of obstructive lung disease and emphysema.
ASTHMA
 Asthma is a chronic inflammatory disorder of the airways in which many inflammatory cells play a role, including mast cells, lymphocytes, neutrophils, and eosinophils. This airway inflammation leads to widespread but variable airflow obstruction that is reversible either spontaneously or with treatment. Asthma is also characterized by increased airway responsiveness to various physiologic and environmental stimuli, such as exercise, cold air, dust mites, and animal dander.
Asthma is a chronic inflammatory disorder of the airways in which many inflammatory cells play a role, including mast cells, lymphocytes, neutrophils, and eosinophils. This airway inflammation leads to widespread but variable airflow obstruction that is reversible either spontaneously or with treatment. Asthma is also characterized by increased airway responsiveness to various physiologic and environmental stimuli, such as exercise, cold air, dust mites, and animal dander.
More than 5% of children under the age of 18 have experienced an asthma attack. Because of its prevalence, the health care burden of asthma is substantial, both in terms of costs and of morbidity. While overall mortality is low and has been relatively stable over the past several decades, specific populations, such as African Americans, have a much higher risk of poor outcomes. Any mortality attributable to asthma is alarming as this is largely preventable with appropriate therapy.
Etiology & Pathogenesis
Much has been learned through autopsy studies on patients who have died with severe asthma. Common features include not only occlusion of the airway by mucous plugging, but also the presence of inflammatory cells, including neutrophils, eosinophils, and lymphocytes. Smooth muscle hypertrophy and hyperplasia are present, along with denuded airway epithelium and subepithelial thickening.
More recently, the pervasiveness of inflammation has been confirmed in bronchial biopsies from patients with mild asthma. While neutrophils are not predominant in these cases, activated eosinophils, mast cells, and lymphocytes are found variably throughout the tracheobronchial tree. Basement membrane collagen deposition and epithelial injury can also be found.
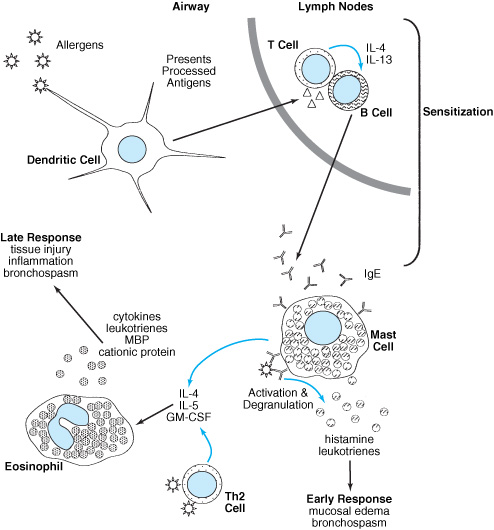
The cycle of inflammation characteristic of asthma begins with sensitization upon inhalation of an allergen. Dendritic cells, which are antigen-presenting cells, migrate to regional lymph nodes where antigen is introduced to resident T and B lymphocytes. The B cells are induced to begin immunoglobulin E (IgE) production by interleukin-4 (IL-4) and interleukin-13 (IL-13) secreted by the T cells. The IgE can then be bound by IgE receptors on airway mast cells (Figure 6–1).
Upon reexposure, the IgE bound to mast cells complexes with the allergen and activates the cell. Activation is followed by the release of histamine, leukotrienes, and cytokines that mediate the physiologic effects of asthma and perpetuate the inflammation.
Among the cytokines produced, several, notably IL-4, IL-5, and granulocyte macrophage-colony stimulating factor, recruit eosinophils to the lung, prolong their survival, and stimulate production of mediators such as major basic protein (MBP) that can injure the bronchial mucosa, induce bronchospasm, and perpetuate the proinflammatory state.
The mechanisms predisposing certain individuals to develop asthma are unknown. Current evidence supports a paradigm referred to as the “hygiene hypothesis.” This theory proposes that environmental exposures early in life dictate the development of immune responses that manifest clinically as allergy and asthma.
Helper CD4+ T cells can be subdivided into Th1-type cells, which produce IL-2 and interferon γ and participate in cell-mediated immunity, or Th2-type cells, which produce IL-4, IL-5, IL-10, and IL-13 and direct allergic inflammation. The hygiene hypothesis suggests that newborns are skewed toward a Th2 phenotype and need early environmental exposures to allow the development of Th1 immunity and to balance the response to future antigen exposure. Both nature and nurture may have a role in the development of asthma. Early life exposure to measles, hepatitis A, infections due to day care contacts, or contact with older siblings, and even in utero presentation of antigens may induce the Th2 → Th1 shift, but the magnitude of the shift is likely influenced by genetic factors.
Figure 6–1. Asthma pathogenesis.
The hereditary aspects of asthma are complex, with over a hundred genes implicated in various studies. Although atopy plays a major role, not all patients demonstrate that clinical phenotype. The presence of rhinitis with or without positive skin tests is associated with a substantial increase in asthma symptoms.
Pathophysiology
Acute allergic asthma has classically been divided into early and late phases. Within minutes of reexposure to a trigger, receptor activation on mast cells induces degranulation and release of histamine, leukotrienes, and other bronchoconstrictors. Smooth muscle contraction and mucosal edema cause the airway obstruction that is responsible for symptomatic asthma. This phase usually resolves within an hour.
A second peak in symptoms, beginning 4–6 hours after exposure and lasting up to 24 hours, characterizes the late response. The symptoms are often more severe. Eosinophil-mediated inflammatory damage is primarily responsible, but other cell types can be involved.
Acute bronchoconstriction and airway edema, along with mucus plug formation, are responsible for increased resistance to airflow. There is narrowing of almost all airways, but small bronchi 2–5 mm in diameter are most affected. Functional residual capacity (FRC) often increases because expiratory times are prolonged and increased inspiratory effort reduces pleural pressure. The increase in FRC places the muscles of respiration at a mechanical disadvantage. These factors increase the work of breathing during an acute attack.
Because of the inhomogeneity of the obstruction, ventilation-perfusion mismatch occurs, compromising gas exchange. In contrast to COPD, asthmatics vigorously compensate for this by hyperventilation. Therefore, although mild to moderate hypoxemia is common, most patients are hypocapnic during exacerbations. The development of hypercapnia signals impending respiratory arrest. With treatment, symptoms can resolve quickly, but abnormalities in pulmonary physiology often persist for weeks.
Despite the episodic nature of the disease with normal spirometry between attacks, asthmatics do not have normal airways between exacerbations. Inflammation can be found even while asymptomatic, and the airways are hyper-responsive to bronchoconstrictor challenge with histamine or methacholine.
It is becoming increasingly clear that unchecked inflammation in asthma has long-term consequences. The chronic inflammatory state leads to deposition of connective tissue and marked basement membrane thickening can occur. This then leads to irreversible airway obstruction and remodeling of airways. An accelerated decline in lung function has been observed in asthma patients, which may be aggravated by tobacco smoking, that can result in clinical and pathophysiologic features similar to COPD.
Diagnostic & Clinical Considerations
Extrinsic, or allergic, asthma usually starts in childhood, and patients have elevated serum IgE levels and positive skin-prick test results for common inhalant allergens. Intrinsic, or nonallergic, asthma typically is of later onset, and patients have negative skin test results for common allergens. Asthma symptoms are variable and tend to follow a circadian rhythm, with the greatest airway narrowing between the hours of 3 and 5 am in the majority of patients.
Typical asthma symptoms include wheezing that is most noticeable on expiration; dyspnea, along with a sensation of chest tightness; and cough secondary to increased airway sensitivity. Cough may actually be the presenting symptom in some patients, particularly children.
The findings on physical examination vary depending on the severity of the episode, but are absent between episodes of asthma. Wheezing is usually heard near the end of expiration in mild asthma, but is present throughout the entire respiratory cycle when the episode is severe (see Chapter 5). The absence of respiratory sounds is indicative of impending respiratory failure and collapse. The respiratory rate is increased, and there is an interruption of speech, as well as the use of accessory muscles of respiration. Tachycardia (higher than 110 beats/min) and pulsus paradoxus (greater than 10 mm Hg difference in blood pressure during inspiration and expiration) are physical findings associated with a severe, acute asthma flare.
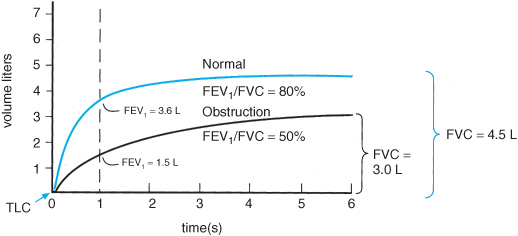
The spirometric changes in obstructive airways disease demonstrate a reduction in the forced expiratory volume in the first second (FEV1) with a low ratio of the FEV1 to the forced vital capacity (FEV1:FVC) (Figure 6–2). However, obstructive physiology is not unique to asthma. Additional supportive evidence of asthma is the reversibility of airflow obstruction demonstrated by a 12% improvement with an absolute increase of 200 mL in the FEV1 after inhalation of a bronchodilator. Complete reversal is expected but not always seen in patients with more severe disease.
Measurement of the peak expiratory flow (PEF) rate is another simple way to assess airflow variability. Its diurnal variation is approximately 15%, it can be measured simply and easily at home, and it may aid in assessing the response to therapy. If confusion remains, a methacholine challenge test can be conducted to determine the degree of airway hyperresponsiveness. The concentration of methacholine required to cause a 20% decline in the FEV1 (PC20) is recorded, and if it is less than 4 mg/mL, the result is considered positive (a PC20 of 4–16 mg/mL is considered borderline).
Figure 6–2. Forced vital capacity (FVC) maneuver using a rolling seal spirometer. Volume–time curves from normal and obstructive subjects.
Management Principles
With appropriate asthma management, the disease can usually be controlled. Asthma must first be viewed as a chronic inflammatory disease, and therefore controlling airway inflammation must be emphasized. The goal is to control the disease so that the patient can function normally. Therapy should be guided by the severity of disease and should include education so the patient may become an active participant in the management of his or her disease. Poorly controlled asthma should be viewed as a failure of therapy, and a change in the treatment plan is warranted. This approach is more important now that chronic inflammation is believed to lead to a progressive decline in airway function.
The recommended pharmacologic therapy for asthma control revolves around a stepwise approach; treatment should be instituted at an intensity expected to control symptoms and then reassessed frequently and modified up or down in a stepwise fashion. If asthma symptoms are intermittent and mild, a short-acting β-agonist on an as-needed basis is all that is typically necessary. Low-dose inhaled corticosteroids should be instituted if asthma symptoms are mild but persistent. Moderate, persistent asthma usually requires the addition of a long-acting inhaled β-agonist bronchodilator. Combination therapy has been found to be more effective than increasing the dose of inhaled steroid. Severe, persistent asthma may necessitate the use of higher inhaled corticosteroid doses, leukotriene inhibitors, theophylline, or even oral steroids. It must be emphasized that scheduled short-acting β-agonists or long-acting β-agonists without concomitant corticosteroids should be avoided as some studies have found an increase in mortality with their use.
Central to the treatment of asthma is trigger avoidance. Short-term triggers, such as exercise and cold air exposure, need not be avoided because they are not believed to alter airway inflammation. Therapy should be maximized so that symptoms are controlled with exercise and airway cooling. It is more important to avoid triggers that lead to chronic airway inflammation, such as dust mites and household pets, since exposure to these triggers can lead to permanent airway changes if inflammation is not adequately controlled. Exposure to known triggers should be controlled more effectively so they no longer cause symptoms.
Patients with known triggers confirmed by allergen testing may be candidates for immunotherapy. Allergen immunotherapy is recommended only for asthmatics who have had a specific allergen identified and have persistent symptoms despite appropriate therapy. Asthmatics with poorly controlled disease may benefit from monoclonal anti-IgE antibody omalizumab trial.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
 COPD is the fourth leading cause of mortality in the United States, and the number of affected individuals is projected to increase worldwide in the future. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as “a disease state characterized by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gases.”
COPD is the fourth leading cause of mortality in the United States, and the number of affected individuals is projected to increase worldwide in the future. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as “a disease state characterized by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gases.”
Etiology & Pathogenesis
Although COPD was frequently subdivided into chronic bronchitis and emphysema in the past, it is now recognized that most patients have elements of both conditions. Chronic bronchitis, which is defined clinically as cough with sputum production for 3 months a year for 2 consecutive years, is associated with hyper-trophy of mucus glands and increased number of goblet cells in the more central airways and peribronchiolar fibrosis in the more peripheral airways. Emphysema is the destruction of alveolar walls and airspace enlargement without significant fibrosis (see Table 6–1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree